Sex-associated hormones and immunity to protozoan parasites
- PMID: 11432809
- PMCID: PMC88985
- DOI: 10.1128/CMR.14.3.476-488.2001
Sex-associated hormones and immunity to protozoan parasites
Abstract
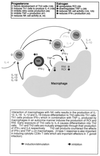
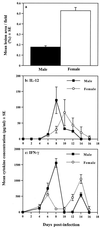
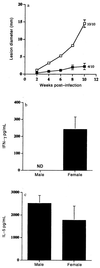
Numerous epidemiological and clinical studies have noted differences in the incidence and severity of parasitic diseases between males and females. Although in some instances this may be due to gender-associated differences in behavior, there is overwhelming evidence that sex-associated hormones can also modulate immune responses and consequently directly influence the outcome of parasitic infection. Animal models of disease can often recreate the gender-dependent differences observed in humans, and the role of sex-associated hormones can be confirmed by experimentally altering their levels. Under normal circumstances, levels of sex hormones not only differ between males and females but vary according to age. Furthermore, not only are females of reproductive age subject to the regular hormonal cycles which control ovulation, they are also exposed to dramatically altered levels during pregnancy. It is thus not surprising that the severity of many diseases, including those caused by parasites, has been shown to be affected by one or more of these circumstances. In addition, infection with many pathogens has been shown to have an adverse influence on pregnancy. In this article we review the impact of sex-associated hormones on the immune system and the development and maintenance of immunity to the intracellular protozoan parasites Toxoplasma gondii, Plasmodium spp., and Leishmania spp.
Figures
References
-
- Ahmed S A, Gogal R M, Walsh J E, Saunders G. Estrogen induces defects in T cell functions of mice. FASEB J. 1996;10:961.
-
- Akingbade O A. Embryotoxic and growth retarding effects of malaria on pregnant mice. J Reprod Med. 1992;37:273–276. - PubMed
-
- Alexander J. Sex differences and cross-immunity in DBA/2 mice infected with L. mexicana and L. major. Parasitology. 1988;96:297–302. - PubMed
-
- Alexander J, Hunter C A. Immunoregulation during toxoplasmosis. Chem Immunol. 1998;70:81–102. - PubMed
-
- Alexander J, Satoskar A R, Russell D G. Leishmania species: models of intracellular parasitism. J Cell Sci. 1999;112:2993–3002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

