Scrapie strains maintain biological phenotypes on propagation in a cell line in culture
- PMID: 11432823
- PMCID: PMC125505
- DOI: 10.1093/emboj/20.13.3351
Scrapie strains maintain biological phenotypes on propagation in a cell line in culture
Abstract
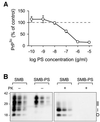
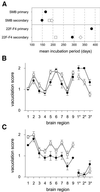
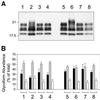
Bovine spongiform encephalopathy (BSE) and its human equivalent, variant Creutzfeldt-Jakob disease (vCJD), are caused by the same strain of infectious agent, which is similar to, but distinct from, >20 strains of their sheep scrapie homologue. A better understanding of the molecular strain determinants could be obtained from cells in monoculture than from whole animal studies where different cell targeting is commonly a strain-related feature. Although a few cell types can be infected with different strains, the phenotypes of the emergent strains have not been studied. We have cured the scrapie-infected, clonal SMB cell line with pentosan sulfate, stably re-infected it with a different strain of scrapie and shown that biological properties and prion protein profiles characteristic of each original strain are propagated faithfully in this single non-neuronal cell type. These findings attest to the fact that scrapie strain determinants are stable and host-independent in isolated cells.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

