Analysis of heterodimer formation by Xklp3A/B, a newly cloned kinesin-II from Xenopus laevis
- PMID: 11432825
- PMCID: PMC125519
- DOI: 10.1093/emboj/20.13.3370
Analysis of heterodimer formation by Xklp3A/B, a newly cloned kinesin-II from Xenopus laevis
Abstract
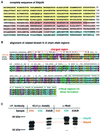
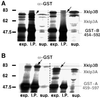
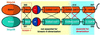
kinesin-II motor proteins are composed of two different kinesin-like motor proteins and one cargo binding subunit. Here we report the cloning of a new member of the kinesin-II superfamily, Xklp3A from Xenopus laevis, which forms a heterodimeric complex with Xklp3B. The heterodimer formation properties between Xklp3A and B have been tested in vitro using reticulocyte lysate expression and immunoprecipitation. To this end we produced a series of Xklp3A and B constructs of varying length and tested their propensity for heterodimer formation. We could demonstrate that, in contrast to conventional kinesin, the critical domains for heterodimer formation in Xklp3A/B are located at the C-terminal end of the stalk. Neither the neck nor the highly charged stretches after the neck region, which are typical of kinesins-II, are required for heterodimer formation, nor do they prevent homodimer formation. Dimerization is controlled by a cooperative mechanism between the C-terminal coiled-coil segments. Classical trigger sites were not identified. The critical regions for dimerization exhibit a very high degree of sequence conservation among equivalent members of the kinesin-II family.
Figures




References
-
- Arndt K.M., Pelletier,J.N., Muller,K.M., Alber,T., Michnick,S.W. and Pluckthun,A. (2000) A heterodimeric coiled-coil peptide pair selected in vivo from a designed library-versus-library ensemble. J. Mol. Biol., 295, 627–639. - PubMed
-
- Brady S.T. (1985) A novel brain ATPase with properties expected for the fast axonal transport motor. Nature, 317, 73–75. - PubMed
-
- Brown J.H., Cohen,C. and Parry,D.A. (1996) Heptad breaks in α-helical coiled coils: stutters and stammers. Proteins, 26, 134–145. - PubMed
-
- Burkhard P., Kammerer,R.A., Steinmetz,M.O., Bourenkov,G.P. and Aebi,U. (2000a) The coiled-coil trigger site of the rod domain of cortexillin I unveils a distinct network of interhelical and intrahelical salt bridges. Struct. Fold. Des., 8, 223–230. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

