Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation
- PMID: 11432826
- PMCID: PMC125511
- DOI: 10.1093/emboj/20.13.3380
Vps45p stabilizes the syntaxin homologue Tlg2p and positively regulates SNARE complex formation
Abstract
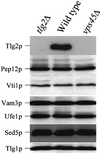
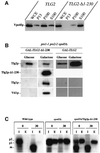
Sec1p-like/Munc-18 (SM) proteins bind to t-SNAREs and inhibit ternary complex formation. Paradoxically, the absence of SM proteins does not result in constitutive membrane fusion. Here, we show that in yeast cells lacking the SM protein Vps45p, the t-SNARE Tlg2p is down-regulated, to undetectable levels, by rapid proteasomal degradation. In the absence of Vps45p, Tlg2p can be stabilized through abolition of proteasome activity. Surprisingly, the stabilized Tlg2p was targeted to the correct intracellular location. However, the stabilized Tlg2p is non-functional and unable to bind its cognate SNARE binding partners, Tlg1p and Vti1p, in the absence of Vps45p. A truncation mutant lacking the first 230 residues of Tlg2p no longer bound Vps45p but was able to form complexes with Tlg1p and Vti1p in the absence of the SM protein. These data provide us with two valuable insights into the function of SM proteins. First, SM proteins act as chaperone-like molecules for their cognate t-SNAREs. Secondly, SM proteins play an essential role in the activation process allowing their cognate t-SNARE to participate in ternary complex formation.
Figures




References
-
- Abeliovich H., Grote,E., Novick,P. and Ferro-Novick,S. (1998) Tlg2p, a yeast syntaxin homolog that resides on the Golgi and endocytic structures. J. Biol. Chem., 273, 11719–11727. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

