Cell-autonomous and non-cell-autonomous functions of the Rb tumor suppressor in developing central nervous system
- PMID: 11432828
- PMCID: PMC125524
- DOI: 10.1093/emboj/20.13.3402
Cell-autonomous and non-cell-autonomous functions of the Rb tumor suppressor in developing central nervous system
Abstract
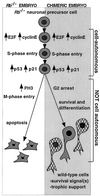
The retinoblastoma tumor suppressor (RB) plays an important role in the regulation of cell cycle progression and terminal differentiation of many cell types. Rb(-/-) mouse embryos die at midgestation with defects in cell cycle regulation, control of apoptosis and terminal differentiation. However, chimeric mice composed of wild-type and Rb-deficient cells are viable and show minor abnormalities. To determine the role of Rb in development more precisely, we analyzed chimeric embryos and adults made with marked Rb(-/-) cells. Like their germline Rb(-/-) counterparts, brains of midgestation chimeric embryos exhibited extensive ectopic S-phase entry. In Rb-mutants, this is accompanied by widespread apoptosis. However, in chimeras, the majority of Rb-deficient cells survived and differentiated into neuronal fates. Rescue of Rb(-/-) neurons in the presence of wild-type cells occurred after induction of the p53 pathway and led to accumulation of cells with 4n DNA content. Therefore, the role of Rb during development can be divided into a cell-autonomous function in exit from the cell cycle and a non-cell-autonomous role in the suppression of apoptosis and induction of differentiation.
Figures

Similar articles
-
Loss of Rb activates both p53-dependent and independent cell death pathways in the developing mouse nervous system.EMBO J. 1996 Nov 15;15(22):6178-88. EMBO J. 1996. PMID: 8947040 Free PMC article.
-
Extra-embryonic function of Rb is essential for embryonic development and viability.Nature. 2003 Feb 27;421(6926):942-7. doi: 10.1038/nature01417. Nature. 2003. PMID: 12607001
-
Telencephalon-specific Rb knockouts reveal enhanced neurogenesis, survival and abnormal cortical development.EMBO J. 2002 Jul 1;21(13):3337-46. doi: 10.1093/emboj/cdf338. EMBO J. 2002. PMID: 12093735 Free PMC article.
-
The retinoblastoma gene family in differentiation and development.Oncogene. 1999 Dec 20;18(55):7873-82. doi: 10.1038/sj.onc.1203244. Oncogene. 1999. PMID: 10630640 Review.
-
Retinoblastoma gene in mouse neural development.Dev Genet. 1996;18(1):81-91. doi: 10.1002/(SICI)1520-6408(1996)18:1<81::AID-DVG9>3.0.CO;2-Y. Dev Genet. 1996. PMID: 8742837 Review.
Cited by
-
Loss of pRB and p107 disrupts cartilage development and promotes enchondroma formation.Oncogene. 2013 Oct;32(40):4798-805. doi: 10.1038/onc.2012.496. Epub 2012 Nov 12. Oncogene. 2013. PMID: 23146901 Free PMC article.
-
Unique requirement for Rb/E2F3 in neuronal migration: evidence for cell cycle-independent functions.Mol Cell Biol. 2007 Jul;27(13):4825-43. doi: 10.1128/MCB.02100-06. Epub 2007 Apr 23. Mol Cell Biol. 2007. PMID: 17452454 Free PMC article.
-
Geminivirus-induced gene silencing of the tobacco retinoblastoma-related gene results in cell death and altered development.Plant Mol Biol. 2007 Sep;65(1-2):163-75. doi: 10.1007/s11103-007-9206-3. Epub 2007 Jul 17. Plant Mol Biol. 2007. PMID: 17634748
-
E2F1 works as a cell cycle suppressor in mature neurons.J Neurosci. 2007 Nov 14;27(46):12555-64. doi: 10.1523/JNEUROSCI.3681-07.2007. J Neurosci. 2007. PMID: 18003834 Free PMC article.
-
A role for the transcription factor HEY1 in glioblastoma.J Cell Mol Med. 2009 Jan;13(1):136-46. doi: 10.1111/j.1582-4934.2008.00307.x. Epub 2008 Mar 17. J Cell Mol Med. 2009. PMID: 18363832 Free PMC article.
References
-
- Bradley A. (1987) Production and analysis of chimeric mice. In Robertson,E.J. (ed.), Teratocarcinomas and Embryonic Stem Cells: A Practical Approach. IRL Press, Oxford, UK, pp. 113–152.
-
- Brehm A. and Kouzarides,T. (1999) Retinoblastoma protein meets chromatin. Trends Biochem. Sci., 24, 142–145. - PubMed
-
- Callaghan D.A., Dong,L., Callaghan,S.M., Hou,Y.X., Dagnino,L. and Slack,R.S. (1999) Neural precursor cells differentiating in the absence of Rb exhibit delayed terminal mitosis and deregulated E2F 1 and 3 activity. Dev. Biol., 207, 257–270. - PubMed
-
- Chen P.L., Riley,D.J., Chen,Y. and Lee,W.H. (1996) Retinoblastoma protein positively regulates terminal adipocyte differentiation through direct interaction with C/EBPs. Genes Dev., 10, 2794–2804. - PubMed
-
- Clarke A.R., Maandag,E.R., van Roon,M., van der Lugt,N.M., van der Valk,M., Hooper,M.L., Berns,A. and te Riele,H. (1992) Requirement for a functional Rb-1 gene in murine development. Nature, 359, 328–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

