A direct interaction between JNK1 and CrkII is critical for Rac1-induced JNK activation
- PMID: 11432831
- PMCID: PMC125507
- DOI: 10.1093/emboj/20.13.3437
A direct interaction between JNK1 and CrkII is critical for Rac1-induced JNK activation
Abstract
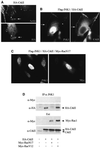
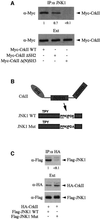
CrkII, a cellular homolog of v-crk, belongs to a family of adaptor proteins that play a central role in signal transduction cascades. We demonstrate that CrkII interacts directly with c-Jun N-terminal kinase 1 (JNK1). A proline-rich sequence of JNK1 is critical for the interaction of the kinase with the N-terminal Src homology 3 (SH3) domain of CrkII. JNK1 is localized with CrkII in membrane ruffles of Crk-overexpressing cells in a Rac1-dependent manner. A JNK1 mutant (K340A) that fails to interact with CrkII is defective in Rac/epidermal growth factor-induced activation, but remains responsive to UVC irradiation. Furthermore, CrkII recruits JNK1 to a p130Cas multiprotein complex where it may be activated through a hematopoietic progenitor kinase 1- and mitogen-activated protein kinase kinase 4-dependent pathway. Together, the results presented here argue for a new mechanism of regulation of the JNK pathway through the CrkII-p130Cas adaptor complex.
Figures



References
-
- Cobb M.H. and Goldsmith,E.J. (1995) How MAP kinases are regulated. J. Biol. Chem., 270, 14843–14846. - PubMed
-
- Coso O.A., Chiariello,M., Yu,J.C., Teramoto,H., Crespo,P., Xu,N., Miki,T. and Gutkind,J.S. (1995) The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell, 81, 1137–1146. - PubMed
-
- Davis R.J. (1999) Signal transduction by the c-Jun N-terminal kinase. Biochem. Soc. Symp., 64, 1–12. - PubMed
-
- Dickens M., Rogers,J.S., Cavanagh,J., Raitano,A., Xia,Z., Halpern,J.R., Greenberg,M.E., Sawyers,C.L. and Davis,R.J. (1997) A cytoplasmic inhibitor of the JNK signal transduction pathway. Science, 277, 693–696. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

