Mixed lineage kinase-dependent JNK activation is governed by interactions of scaffold protein JIP with MAPK module components
- PMID: 11432832
- PMCID: PMC125504
- DOI: 10.1093/emboj/20.13.3447
Mixed lineage kinase-dependent JNK activation is governed by interactions of scaffold protein JIP with MAPK module components
Abstract
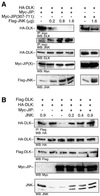
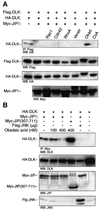
It has been proposed that JNK-interacting proteins (JIP) facilitate mixed lineage kinase-dependent signal transduction to JNK by aggregating the three components of a JNK module. A new model for the assembly and regulation of these modules is proposed based on several observations. First, artificially induced dimerization of dual leucine zipper-bearing kinase (DLK) confirmed that DLK dimerization is sufficient to induce DLK activation. Secondly, under basal conditions, DLK associated with JIP is held in a monomeric, unphosphorylated and catalytically inactive state. Thirdly, JNK recruitment to JIP coincided with significantly decreased affinity of JIP and DLK. JNK promoted the dimerization, phosphorylation and activation of JIP-associated DLK. Similarly, treatment of cells with okadaic acid inhibited DLK association with JIP and resulted in DLK dimerization in the presence of JIP. In summary, JIP maintains DLK in a monomeric, unphosphorylated, inactive state. Upon stimulation, JNK-JIP binding affinity increases while JIP-DLK interaction affinity is attenuated. Dissociation of DLK from JIP results in subsequent DLK dimerization, autophosphorylation and module activation. Evidence is provided that this model holds for other MLK-dependent JNK modules.
Figures








Similar articles
-
Recruitment of JNK to JIP1 and JNK-dependent JIP1 phosphorylation regulates JNK module dynamics and activation.J Biol Chem. 2003 Aug 1;278(31):28694-702. doi: 10.1074/jbc.M304212200. Epub 2003 May 19. J Biol Chem. 2003. PMID: 12756254
-
Identification of structural and functional domains in mixed lineage kinase dual leucine zipper-bearing kinase required for complex formation and stress-activated protein kinase activation.J Biol Chem. 2000 Mar 10;275(10):7273-9. doi: 10.1074/jbc.275.10.7273. J Biol Chem. 2000. PMID: 10702297
-
Mixed lineage kinase LZK forms a functional signaling complex with JIP-1, a scaffold protein of the c-Jun NH(2)-terminal kinase pathway.J Biochem. 2001 Dec;130(6):773-81. doi: 10.1093/oxfordjournals.jbchem.a003048. J Biochem. 2001. PMID: 11726277
-
[Regulation of the JNK signaling pathway by dual leucine zipper kinase DLK.].Yi Chuan. 2010 Aug;32(8):785-90. doi: 10.3724/sp.j.1005.2010.00785. Yi Chuan. 2010. PMID: 20709675 Review. Chinese.
-
From receptors to stress-activated MAP kinases.Oncogene. 1999 Nov 1;18(45):6087-93. doi: 10.1038/sj.onc.1203129. Oncogene. 1999. PMID: 10557099 Review.
Cited by
-
A unique set of SH3-SH3 interactions controls IB1 homodimerization.EMBO J. 2006 Feb 22;25(4):785-97. doi: 10.1038/sj.emboj.7600982. Epub 2006 Feb 2. EMBO J. 2006. PMID: 16456539 Free PMC article.
-
Inactivating Celsr2 promotes motor axon fasciculation and regeneration in mouse and human.Brain. 2022 Apr 18;145(2):670-683. doi: 10.1093/brain/awab317. Brain. 2022. PMID: 34983065 Free PMC article.
-
Obesity-induced TRB3 negatively regulates Brown adipose tissue function in mice.Biochem Biophys Res Commun. 2021 Apr 2;547:29-35. doi: 10.1016/j.bbrc.2021.01.103. Epub 2021 Feb 13. Biochem Biophys Res Commun. 2021. PMID: 33592376 Free PMC article.
-
MLK3 regulates fMLP-stimulated neutrophil motility.Mol Immunol. 2014 Apr;58(2):214-22. doi: 10.1016/j.molimm.2013.11.016. Epub 2014 Jan 3. Mol Immunol. 2014. PMID: 24389043 Free PMC article.
-
ZPK/DLK and MKK4 form the critical gateway to axotomy-induced motoneuron death in neonates.J Neurosci. 2014 Aug 6;34(32):10729-42. doi: 10.1523/JNEUROSCI.0539-14.2014. J Neurosci. 2014. PMID: 25100604 Free PMC article.
References
-
- Barancik M., Htun,P. and Schaper,W. (1999) Okadaic acid and anisomycin are protective and stimulate the SAPK/JNK pathway. J. Cardiovasc. Pharmacol., 34, 182–190. - PubMed
-
- Bock B.C., Vacratsis,P.O., Qamirani,E. and Gallo,K.A. (2000) Cdc42-induced activation of the mixed-lineage kinase SPRK in vivo. Requirement of the Cdc42/Rac interactive binding motif and changes in phosphorylation. J. Biol. Chem., 275, 14231–14241. - PubMed
-
- Burack R.W. and Shaw,A.S. (2000) Signal transduction: hanging on scaffold. Curr. Opin. Cell Biol., 12, 211–216. - PubMed
-
- Chen Y.R., Meyer,C.F. and Tan,T.H. (1996) Persistent activation of c-Jun N-terminal kinase 1 (JNK1) in γ radiation-induced apoptosis. J. Biol. Chem., 271, 631–634. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

