Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1
- PMID: 11432833
- PMCID: PMC125530
- DOI: 10.1093/emboj/20.13.3459
Pin1 is overexpressed in breast cancer and cooperates with Ras signaling in increasing the transcriptional activity of c-Jun towards cyclin D1
Abstract
Phosphorylation on serines or threonines preceding proline (Ser/Thr-Pro) is a major signaling mechanism. The conformation of a subset of phosphorylated Ser/Thr-Pro motifs is regulated by the prolyl isomerase Pin1. Inhibition of Pin1 induces apoptosis and may also contribute to neuronal death in Alzheimer's disease. However, little is known about the role of Pin1 in cancer or in modulating transcription factor activity. Here we report that Pin1 is strikingly overexpressed in human breast cancers, and that its levels correlate with cyclin D1 levels in tumors. Overexpression of Pin1 increases cellular cyclin D1 protein and activates its promoter. Furthermore, Pin1 binds c-Jun that is phosphorylated on Ser63/73-Pro motifs by activated JNK or oncogenic Ras. Moreover, Pin1 cooperates with either activated Ras or JNK to increase transcriptional activity of c-Jun towards the cyclin D1 promoter. Thus, Pin1 is up-regulated in human tumors and cooperates with Ras signaling in increasing c-Jun transcriptional activity towards cyclin D1. Given the crucial roles of Ras signaling and cyclin D1 overexpression in oncogenesis, our results suggest that overexpression of Pin1 may promote tumor growth.
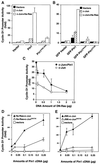
Figures






References
-
- Albanese C. et al. (1999) Activation of the cyclin D1 gene by the E1A-associated protein p300 through AP-1 inhibits cellular apoptosis. J. Biol. Chem., 274, 34186–34195. - PubMed
-
- Albanese C., Johnson,J., Watanabe,G., Eklund,N., Vu,D., Arnold,A. and Pestell,R.G. (1995) Transforming p21ras mutants and c-Ets-2 activate the cyclin D1 promoter through distinguishable regions. J. Biol. Chem., 270, 23589–23597. - PubMed
-
- Angel P., Smeal,T., Meek,J. and Karin,M. (1989) Jun and v-jun contain multiple regions that participate in transcriptional activation in an interdependent manner. New Biol., 1, 35–43. - PubMed
-
- Arber N. et al. (1997) Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res., 57, 1569–1574. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

