Double-check probing of DNA bending and unwinding by XPA-RPA: an architectural function in DNA repair
- PMID: 11432842
- PMCID: PMC125508
- DOI: 10.1093/emboj/20.13.3554
Double-check probing of DNA bending and unwinding by XPA-RPA: an architectural function in DNA repair
Abstract
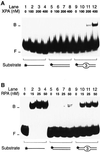
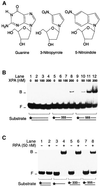
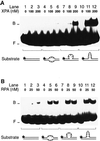
The multiprotein factor composed of XPA and replication protein A (RPA) is an essential subunit of the mammalian nucleotide excision repair system. Although XPA-RPA has been implicated in damage recognition, its activity in the DNA repair pathway remains controversial. By replacing DNA adducts with mispaired bases or non-hybridizing analogues, we found that the weak preference of XPA and RPA for damaged substrates is entirely mediated by indirect readout of DNA helix conformations. Further screening with artificially distorted substrates revealed that XPA binds most efficiently to rigidly bent duplexes but not to single-stranded DNA. Conversely, RPA recognizes single-stranded sites but not backbone bending. Thus, the association of XPA with RPA generates a double-check sensor that detects, simultaneously, backbone and base pair distortion of DNA. The affinity of XPA for sharply bent duplexes, characteristic of architectural proteins, is not compatible with a direct function during recognition of nucleotide lesions. Instead, XPA in conjunction with RPA may constitute a regulatory factor that monitors DNA bending and unwinding to verify the damage-specific localization of repair complexes or control their correct three-dimensional assembly.
Figures





References
-
- Aboussekhra A. et al. (1995) Mammalian DNA nucleotide excision repair reconstituted with purified components. Cell, 80, 859–868. - PubMed
-
- Araújo S.J., Tirode,F., Coin,F., Pospiech,H., Syväoja,J.E., Stucki,M., Hübscher,U., Egly,J.-M. and Wood,R.D. (2000) Nucleotide excision repair of DNA with recombinant human proteins: definition of the minimal set of factors, active forms of TFIIH, and modulation by CAK. Genes Dev., 14, 349–359. - PMC - PubMed
-
- Asahina H., Kuraoka,I., Shirakawa,M., Morita,E.H., Miura,N., Miyamoto,I., Ohtsuka,E., Okada,Y. and Tanaka,K. (1994) The XPA protein is a zinc metalloprotein with an ability to recognize various kinds of DNA damage. Mutat. Res., 315, 229–237. - PubMed
-
- Batty D., Rapic’-Otrin,V., Levine,A.S. and Wood,R.D. (2000) Stable binding of human XPC complex to irradiated DNA confers strong discrimination for damaged sites. J. Mol. Biol., 300, 275–290. - PubMed
-
- Burns J.L., Guzder,S.N., Sung,P., Prakash,S. and Prakash,L. (1996) An affinity of human replication protein A for ultraviolet-damaged DNA. J. Biol. Chem., 271, 11607–11610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

