Human immunodeficiency virus type 1 integrase: arrangement of protein domains in active cDNA complexes
- PMID: 11432843
- PMCID: PMC125503
- DOI: 10.1093/emboj/20.13.3565
Human immunodeficiency virus type 1 integrase: arrangement of protein domains in active cDNA complexes
Abstract
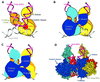
Early steps of retroviral replication involve reverse transcription of the viral RNA genome and integration of the resulting cDNA copy into a chromosome of the host cell. The viral-encoded integrase protein carries out the initial DNA breaking and joining reactions that mediate integration. The organization of the active integrase-DNA complex is unknown, though integrase is known to act as a multimer, and high resolution structures of the isolated integrase domains have been determined. Here we use site-specific cross-linking based on disulfide bond formation to map integrase-DNA contacts in active complexes. We establish that the DNA-binding C-terminal domain of one integrase monomer acts with the central catalytic domain from another monomer at each viral cDNA end. These data allow detailed modeling of an integrase tetramer in which pairs of trans interactions link integrase dimers bound to substrate DNA. We also detected a conformational change in integrase- DNA complexes accompanying cleavage of the viral cDNA terminus.
Figures


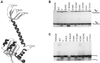

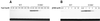

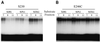
References
-
- Aldaz H., Schuster,E. and Baker,T.A. (1996) The interwoven architecture of the Mu transposase couples DNA synapsis to catalysis. Cell, 85, 257–269. - PubMed
-
- Asante-Appiah E., Seeholzer,S.H. and Skalka,A.M. (1998) Structural determinants of metal-induced conformational changes in HIV-1 integrase. J. Biol. Chem., 273, 35078–35087. - PubMed
-
- Bujacz G., Jaskolski,M., Alexandratos,J., Wlodawer,A., Merkel,G., Katz,R.A. and Skalka,A.M. (1995) High-resolution structure of the catalytic domain of avian sarcoma virus integrase. J. Mol. Biol., 253, 333–346. - PubMed
-
- Bushman F.D., Fujiwara,T. and Craigie,R. (1990) Retroviral DNA integration directed by HIV integration protein in vitro. Science, 249, 1555–1558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

