Effects of membrane cholesterol manipulation on excitation-contraction coupling in skeletal muscle of the toad
- PMID: 11432993
- PMCID: PMC2278681
- DOI: 10.1111/j.1469-7793.2001.00071.x
Effects of membrane cholesterol manipulation on excitation-contraction coupling in skeletal muscle of the toad
Abstract
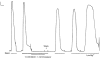
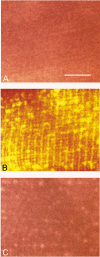
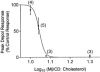
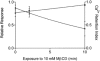
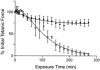
1. Single mechanically skinned fibres and intact bundles of fibres from the twitch region of the iliofibularis muscle of cane toads were used to investigate the effects of membrane cholesterol manipulation on excitation-contraction (E-C) coupling. The cholesterol content of membranes was manipulated with methyl-beta-cyclodextrin (MbetaCD). 2. In mechanically skinned fibres, depletion of membrane cholesterol with MbetaCD caused a dose- and time-dependent decrease in transverse tubular (t)-system depolarization-induced force responses (TSDIFRs). TSDIFRs were completely abolished within 2 min in the presence of 10 mM MbetaCD but were not affected after 2 min in the presence of a 10 mM MbetaCD-1 mM cholesterol complex. There was a very steep dependence between the change in TSDIFRs and the MbetaCD : cholesterol ratio at 10 mM MbetaCD, indicating that the inhibitory effect of MbetaCD was due to membrane cholesterol depletion and not to a pharmacological effect of the agent. Tetanic responses in bundles of intact fibres were abolished after 3-4 h in the presence of 10 mM MbetaCD. 3. The duration of TSDIFRs increased markedly soon (< 2 min) after application of 10 mM MbetaCD and 10 mM MbetaCD-cholesterol complexes, but the Ca(2+) activation properties of the contractile apparatus were minimally affected by 10 mM MbetaCD. The Ca(2+) handling abilities of the sarcoplasmic reticulum appeared to be modified after 10 min exposure to 10 mM MbetaCD. 4. Confocal laser scanning microscopy revealed that the integrity of the t-system was not compromised by either intra- or extracellular application of 10 mM MbetaCD and that a large [Ca(2+)] gradient was maintained across the t-system. 5. Membrane cholesterol depletion caused rapid depolarization of the polarized t-system as shown independently by spontaneous TSDIFRs induced by MbetaCD and by changes in the fluorescence intensity of an anionic potentiometric dye (DiBAC(4)(3)) in the presence of MbetaCD. This rapid depolarization of the t-system by cholesterol depletion was not prevented by blocking the Na(+) channels with TTX (10 microM) or the L-type Ca(2+) channels with Co(2+) (5 mM). 6. The results demonstrate that cholesterol is important for maintaining the functional integrity of the t-system and sarcoplasmic reticulum, probably by having specific effects on different membrane proteins that may be directly or indirectly involved in E-C coupling.
Figures
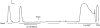


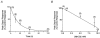
Similar articles
-
The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad.J Muscle Res Cell Motil. 1996 Feb;17(1):55-67. doi: 10.1007/BF00140324. J Muscle Res Cell Motil. 1996. PMID: 8740432
-
Effects of reducing agents and oxidants on excitation-contraction coupling in skeletal muscle fibres of rat and toad.J Physiol. 1996 Nov 1;496 ( Pt 3)(Pt 3):809-25. doi: 10.1113/jphysiol.1996.sp021729. J Physiol. 1996. PMID: 8930846 Free PMC article.
-
Glycogen content and excitation-contraction coupling in mechanically skinned muscle fibres of the cane toad.J Physiol. 1999 Aug 15;519 Pt 1(Pt 1):177-87. doi: 10.1111/j.1469-7793.1999.0177o.x. J Physiol. 1999. PMID: 10432348 Free PMC article.
-
"Current" advances in mechanically skinned skeletal muscle fibres.Clin Exp Pharmacol Physiol. 2001 Aug;28(8):668-74. doi: 10.1046/j.1440-1681.2001.03502.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11473535 Review.
-
Measurement of force and calcium release using mechanically skinned fibers from mammalian skeletal muscle.J Appl Physiol (1985). 2018 Oct 1;125(4):1105-1127. doi: 10.1152/japplphysiol.00445.2018. Epub 2018 Jul 19. J Appl Physiol (1985). 2018. PMID: 30024333 Review.
Cited by
-
Identification of the coupling between skeletal muscle store-operated Ca2+ entry and the inositol trisphosphate receptor.Proc Natl Acad Sci U S A. 2003 Mar 4;100(5):2941-4. doi: 10.1073/pnas.0536227100. Epub 2003 Feb 24. Proc Natl Acad Sci U S A. 2003. PMID: 12601149 Free PMC article.
-
Diverse presynaptic mechanisms underlying methyl-β-cyclodextrin-mediated changes in glutamate transport.Cell Mol Neurobiol. 2010 Oct;30(7):1013-23. doi: 10.1007/s10571-010-9532-x. Epub 2010 May 26. Cell Mol Neurobiol. 2010. PMID: 20502957 Free PMC article.
-
Membrane Perfusion of Hydrophobic Substances Around Channels Embedded in the Contact Bubble Bilayer.Sci Rep. 2017 Jul 31;7(1):6857. doi: 10.1038/s41598-017-07048-4. Sci Rep. 2017. PMID: 28761089 Free PMC article.
-
Cholesterol and synaptic transmitter release at crayfish neuromuscular junctions.J Physiol. 2006 Feb 15;571(Pt 1):83-99. doi: 10.1113/jphysiol.2005.098319. Epub 2005 Dec 8. J Physiol. 2006. PMID: 16339182 Free PMC article.
-
Cholesterol depletion from the plasma membrane impairs proton and glutamate storage in synaptic vesicles of nerve terminals.J Mol Neurosci. 2010 Jul;41(3):358-67. doi: 10.1007/s12031-010-9351-z. Epub 2010 Apr 6. J Mol Neurosci. 2010. PMID: 20369388
References
-
- Aepfelbacher M, Hrboticky N, Lux I, Weber PC. Cholesterol modulates PAF-stimulated Ca2+-mobilization in monocytic U937 cells. Biochimica et Biophysica Acta. 1991;1074:125–129. - PubMed
-
- Anderson R G W. The caveolae membrane system. Annual Review of Biochemistry. 1998;67:199–225. - PubMed
-
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. Journal of Muscle Research and Cell Motility. 1996;17:55–67. - PubMed
-
- Bangham AD, Horne RW. Action of saponin on biological cell membranes. Nature. 1962;196:952–953. - PubMed
-
- Bastiaanse E M L, Astma DE, Kuijpers M M C, VanderLaarse A. The effect of sarcolemmal cholesterol content on intracellular calcium ion concentration in cultured cardiomyocytes. Archives of Biochemistry and Biophysics. 1994;313:58–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

