The use of the indicator fluo-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad
- PMID: 11432994
- PMCID: PMC2278698
- DOI: 10.1111/j.1469-7793.2001.00087.x
The use of the indicator fluo-5N to measure sarcoplasmic reticulum calcium in single muscle fibres of the cane toad
Abstract
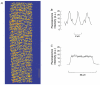
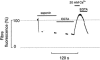
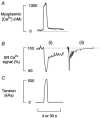
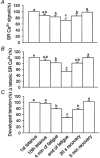
1. Single fibres from the lumbrical muscles of the cane toad (Bufo marinus) were incubated in fluo-5N AM for 2 h at 35 degrees C in order to load the indicator into the sarcoplasmic reticulum. Fluo-5N is a low-affinity calcium indicator (K(Ca) 90 microM). Successful sarcoplasmic reticulum (SR) loading was indicated by a fluorescence signal that declined during contraction. 2. Confocal microscopy showed that the dye loaded principally in lines perpendicular to the long axis of the fibre that repeated each sarcomere. This is consistent with much of the dye residing in the SR. 3. To establish the site of loading, fibres were exposed to 30 mM caffeine in the presence of 20 microM 2,5-di(tert-butyl)1,4-hydroquinone (TBQ, an SR pump inhibitor) which should release most Ca(2+) from the SR; this procedure reduced the fluorescence to 46 +/- 4 % of the control value. To determine how much indicator was in the myoplasm, fibres were exposed to 100 microg ml(-1) saponin which permeabilizes the surface membrane; saponin treatment reduced the fluorescence to 51 +/- 2 % of the control value. 4. During maximally activated tetani (100 Hz stimulation rate, 22 degrees C) the component of signal from the SR declined by 33 +/- 4 %. During relaxation the SR signal recovered in two phases with time constants of 0.38 +/- 0.14 s and 10.1 +/- 1.7 s. Partially activated tetani (30 Hz stimulation rate) showed a smaller SR signal. Application of the SR Ca(2+) pump inhibitor TBQ slowed the rate of recovery of the SR signal. 5. Muscle fatigue was produced by repeated short tetani until tension was reduced to 50 %. The SR signal during the periods between tetani declined steadily and the SR Ca(2+) signal was eventually reduced to 71 +/- 8 % of the control signal. This signal recovered in two phases when the muscle was rested. An initial phase had a time constant of 1.7 +/- 0.2 s so that by 20 s of recovery the SR Ca(2+) signal was 86 +/- 7 % of control; the second phase was slower and by 5 min the SR Ca(2+) signal was back to control values (98 +/- 5 % control). In addition the magnitude of the SR signal decline associated with each tetanus (Delta[Ca(2+)](SR)) declined monotonically throughout fatigue and returned to control after 5 min recovery. 6. This approach can monitor the SR Ca(2+) concentration in normally functioning muscle fibres with good time resolution. The method confirms other approaches that show that the free Ca(2+) available for release in the SR declines during fatigue. This reduction in [Ca(2+)](SR) will contribute to the failure of Ca(2+) delivery to the myofilaments which is an important cause of muscle fatigue.
Figures



Similar articles
-
The role of calcium stores in fatigue of isolated single muscle fibres from the cane toad.J Physiol. 1999 Aug 15;519 Pt 1(Pt 1):169-76. doi: 10.1111/j.1469-7793.1999.0169o.x. J Physiol. 1999. PMID: 10432347 Free PMC article.
-
Intracellular calcium during fatigue of cane toad skeletal muscle in the absence of glucose.J Muscle Res Cell Motil. 2000;21(5):481-9. doi: 10.1023/a:1005650425513. J Muscle Res Cell Motil. 2000. PMID: 11129439
-
Effect of saponin treatment on the sarcoplasmic reticulum of rat, cane toad and crustacean (yabby) skeletal muscle.J Physiol. 1997 Oct 15;504 ( Pt 2)(Pt 2):425-37. doi: 10.1111/j.1469-7793.1997.425be.x. J Physiol. 1997. PMID: 9365915 Free PMC article.
-
A study of the mechanisms of excitation-contraction coupling in frog skeletal muscle based on measurements of [Ca2+] transients inside the sarcoplasmic reticulum.J Muscle Res Cell Motil. 2018 Apr;39(1-2):41-60. doi: 10.1007/s10974-018-9497-9. Epub 2018 Aug 24. J Muscle Res Cell Motil. 2018. PMID: 30143958 Review.
-
Impaired calcium release during fatigue.J Appl Physiol (1985). 2008 Jan;104(1):296-305. doi: 10.1152/japplphysiol.00908.2007. Epub 2007 Oct 25. J Appl Physiol (1985). 2008. PMID: 17962573 Review.
Cited by
-
Novel approach for quantification of endoplasmic reticulum Ca2+ transport.Am J Physiol Heart Circ Physiol. 2019 Jun 1;316(6):H1323-H1331. doi: 10.1152/ajpheart.00031.2019. Epub 2019 Mar 22. Am J Physiol Heart Circ Physiol. 2019. PMID: 30901276 Free PMC article.
-
Dynamic local changes in sarcoplasmic reticulum calcium: physiological and pathophysiological roles.J Mol Cell Cardiol. 2012 Feb;52(2):304-11. doi: 10.1016/j.yjmcc.2011.06.024. Epub 2011 Jul 13. J Mol Cell Cardiol. 2012. PMID: 21767546 Free PMC article. Review.
-
Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca(2+) release in skeletal muscle.J Gen Physiol. 2011 Aug;138(2):231-47. doi: 10.1085/jgp.201010592. J Gen Physiol. 2011. PMID: 21788611 Free PMC article.
-
Shiga toxin 1-induced proinflammatory cytokine production is regulated by the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin signaling pathway.Infect Immun. 2009 Sep;77(9):3919-31. doi: 10.1128/IAI.00738-09. Epub 2009 Jul 13. Infect Immun. 2009. PMID: 19596774 Free PMC article.
-
SERCA2a upregulation ameliorates cellular alternans induced by metabolic inhibition.J Appl Physiol (1985). 2016 Apr 15;120(8):865-75. doi: 10.1152/japplphysiol.00588.2015. Epub 2016 Feb 4. J Appl Physiol (1985). 2016. PMID: 26846549 Free PMC article.
References
-
- Allen DG, Lännergren J, Westerblad H. Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Experimental Physiology. 1995;80:497–527. - PubMed
-
- Balnave CD, Allen DG. Evidence for Na+/Ca2+ exchange in intact single skeletal muscle fibers from the mouse. American Journal of Physiology. 1998;274:C940–946. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

