The Bloom's and Werner's syndrome proteins are DNA structure-specific helicases
- PMID: 11433031
- PMCID: PMC55766
- DOI: 10.1093/nar/29.13.2843
The Bloom's and Werner's syndrome proteins are DNA structure-specific helicases
Abstract
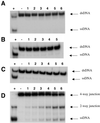
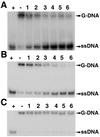
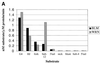
BLM and WRN, the products of the Bloom's and Werner's syndrome genes, are members of the RecQ family of DNA helicases. Although both have been shown previously to unwind simple, partial duplex DNA substrates with 3'-->5' polarity, little is known about the structural features of DNA that determine the substrate specificities of these enzymes. We have compared the substrate specificities of the BLM and WRN proteins using a variety of partial duplex DNA molecules, which are based upon a common core nucleotide sequence. We show that neither BLM nor WRN is capable of unwinding duplex DNA from a blunt-ended terminus or from an internal nick. However, both enzymes efficiently unwind the same blunt-ended duplex containing a centrally located 12 nt single-stranded 'bubble', as well as a synthetic X-structure (a model for the Holliday junction recombination intermediate) in which each 'arm' of the 4-way junction is blunt-ended. Surprisingly, a 3'-tailed duplex, a standard substrate for 3'-->5' helicases, is unwound much less efficiently by BLM and WRN than are the bubble and X-structure substrates. These data show conclusively that a single-stranded 3'-tail is not a structural requirement for unwinding of standard B-form DNA by these helicases. BLM and WRN also both unwind a variety of different forms of G-quadruplex DNA, a structure that can form at guanine-rich sequences present at several genomic loci. Our data indicate that BLM and WRN are atypical helicases that are highly DNA structure specific and have similar substrate specificities. We interpret these data in the light of the genomic instability and hyper-recombination characteristics of cells from individuals with Bloom's or Werner's syndrome.
Figures



References
-
- Lohman T.M. and Bjornson,K.P. (1996) Mechanisms of helicase-catalyzed DNA unwinding. Annu. Rev. Biochem., 65, 169–214. - PubMed
-
- Chakraverty R.K. and Hickson,I.D. (1999) Defending genome integrity during DNA replication: a proposed role for RecQ family helicases. Bioessays, 21, 286–294. - PubMed
-
- Karow J.K., Wu,L. and Hickson,I.D. (2000) RecQ family helicases: roles in cancer and aging. Curr. Opin. Genet. Dev., 10, 32–38. - PubMed
-
- Seki M., Miyazawa,H., Tada,S., Yanagisawa,J., Yamaoka,T., Hoshino,S., Ozawa,K., Eki,T., Nogami,M., Okumura,K. et al. (1994) Molecular cloning of cDNA encoding human DNA helicase Q1 which has homology to Escherichia coli Rec Q helicase and localization of the gene at chromosome 12p12. Nucleic Acids Res., 22, 4566–4573. - PMC - PubMed
-
- Puranam K.L. and Blackshear,P.J. (1994) Cloning and characterization of RECQL, a potential human homologue of the Escherichiacoli DNA helicase RecQ. J. Biol. Chem., 269, 29838–29845. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

