Translesion synthesis by yeast DNA polymerase zeta from templates containing lesions of ultraviolet radiation and acetylaminofluorene
- PMID: 11433034
- PMCID: PMC55783
- DOI: 10.1093/nar/29.13.2875
Translesion synthesis by yeast DNA polymerase zeta from templates containing lesions of ultraviolet radiation and acetylaminofluorene
Abstract
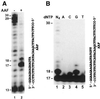
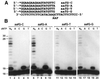
In the yeast Saccharomyces cerevisiae, DNA polymerase zeta (Polzeta) is required in a major lesion bypass pathway. To help understand the role of Polzeta in lesion bypass, we have performed in vitro biochemical analyses of this polymerase in response to several DNA lesions. Purified yeast Polzeta performed limited translesion synthesis opposite a template TT (6-4) photoproduct, incorporating A or T with similar efficiencies (and less frequently G) opposite the 3' T, and predominantly A opposite the 5' T. Purified yeast Polzeta predominantly incorporated a G opposite an acetylaminofluorene (AAF)-adducted guanine. The lesion, however, significantly inhibited subsequent extension. Furthermore, yeast Polzeta catalyzed extension DNA synthesis from primers annealed opposite the AAF-guanine and the 3' T of the TT (6-4) photoproduct with varying efficiencies. Extension synthesis was more efficient when A or C was opposite the AAF-guanine, and when G was opposite the 3' T of the TT (6-4) photoproduct. In contrast, the 3' T of a cis-syn TT dimer completely blocked purified yeast Polzeta, whereas the 5' T was readily bypassed. These results support the following dual-function model of Polzeta. First, Polzeta catalyzes nucleotide incorporation opposite AAF-guanine and TT (6-4) photoproduct with a limited efficiency. Secondly, more efficient bypass of these lesions may require nucleotide incorporation by other DNA polymerases followed by extension DNA synthesis by Polzeta.
Figures

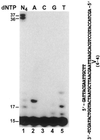
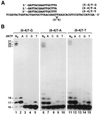
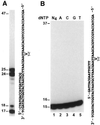
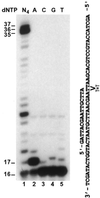
Similar articles
-
Translesion synthesis of acetylaminofluorene-dG adducts by DNA polymerase zeta is stimulated by yeast Rev1 protein.Nucleic Acids Res. 2004 Feb 11;32(3):1122-30. doi: 10.1093/nar/gkh279. Print 2004. Nucleic Acids Res. 2004. PMID: 14960722 Free PMC article.
-
Error-free and error-prone lesion bypass by human DNA polymerase kappa in vitro.Nucleic Acids Res. 2000 Nov 1;28(21):4138-46. doi: 10.1093/nar/28.21.4138. Nucleic Acids Res. 2000. PMID: 11058110 Free PMC article.
-
Response of human DNA polymerase iota to DNA lesions.Nucleic Acids Res. 2001 Feb 15;29(4):928-35. doi: 10.1093/nar/29.4.928. Nucleic Acids Res. 2001. PMID: 11160925 Free PMC article.
-
Eukaryotic mutagenesis and translesion replication dependent on DNA polymerase zeta and Rev1 protein.Biochem Soc Trans. 2001 May;29(Pt 2):187-91. doi: 10.1042/0300-5127:0290187. Biochem Soc Trans. 2001. PMID: 11356151 Review.
-
Eukaryotic translesion synthesis DNA polymerases: specificity of structure and function.Annu Rev Biochem. 2005;74:317-53. doi: 10.1146/annurev.biochem.74.082803.133250. Annu Rev Biochem. 2005. PMID: 15952890 Review.
Cited by
-
Analysis of CPD ultraviolet lesion bypass in chicken DT40 cells: polymerase η and PCNA ubiquitylation play identical roles.PLoS One. 2012;7(12):e52472. doi: 10.1371/journal.pone.0052472. Epub 2012 Dec 18. PLoS One. 2012. PMID: 23272247 Free PMC article.
-
Mutational analysis of the C8-guanine adduct of the environmental carcinogen 3-nitrobenzanthrone in human cells: critical roles of DNA polymerases η and κ and Rev1 in error-prone translesion synthesis.Biochemistry. 2014 Aug 19;53(32):5323-31. doi: 10.1021/bi5007805. Epub 2014 Aug 6. Biochemistry. 2014. PMID: 25080294 Free PMC article.
-
Mutagenic bypass of the butadiene-derived 2'-deoxyuridine adducts by polymerases eta and zeta.Mutat Res. 2007 Dec 1;625(1-2):40-9. doi: 10.1016/j.mrfmmm.2007.05.003. Epub 2007 May 18. Mutat Res. 2007. PMID: 17586533 Free PMC article.
-
Participation of DNA polymerase zeta in replication of undamaged DNA in Saccharomyces cerevisiae.Genetics. 2010 Jan;184(1):27-42. doi: 10.1534/genetics.109.107482. Epub 2009 Oct 19. Genetics. 2010. PMID: 19841096 Free PMC article.
-
The fidelity of DNA synthesis by yeast DNA polymerase zeta alone and with accessory proteins.Nucleic Acids Res. 2006;34(17):4731-42. doi: 10.1093/nar/gkl465. Epub 2006 Sep 13. Nucleic Acids Res. 2006. PMID: 16971464 Free PMC article.
References
-
- Reuven N.B., Arad,G., Maor-Shoshani,A. and Livneh,Z. (1999) The mutagenesis protein UmuC is a DNA polymerase activated by UmuD′, RecA and SSB and is specialized for translesion replication. J. Biol. Chem., 274, 31763–31766. - PubMed
-
- Tang M., Pham,P., Shen,X., Taylor,J.S., O’Donnell,M., Woodgate,R. and Goodman,M.F. (2000) Roles of E. coli DNA polymerases IV and V in lesion-targeted and untargeted SOS mutagenesis. Nature, 404, 1014–1018. - PubMed
-
- Johnson R.E., Prakash,S. and Prakash,L. (1999) Efficient bypass of a thymine–thymine dimer by yeast DNA polymerase, Polη. Science, 283, 1001–1004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

