Metalloproteinases in biology and pathology of the nervous system
- PMID: 11433375
- PMCID: PMC7097548
- DOI: 10.1038/35081571
Metalloproteinases in biology and pathology of the nervous system
Abstract
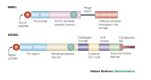
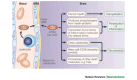
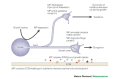
Matrix metalloproteinases (MMPs) have been implicated in several diseases of the nervous system. Here we review the evidence that supports this idea and discuss the possible mechanisms of MMP action. We then consider some of the beneficial functions of MMPs during neural development and speculate on their roles in repair after brain injury. We also introduce a family of proteins known as ADAMs (a disintegrin and metalloproteinase), as some of the properties previously ascribed to MMPs are possibly the result of ADAM activity.
Figures
References
-
- Schlondorff J, Blobel CP. Metalloprotease-disintegrins: modular proteins capable of promoting cell–cell interactions and triggering signals by protein-ectodomain shedding. J. Cell Sci. 1999;112:3603–3617. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

