Expression of the oxygen-regulated protein ORP150 accelerates wound healing by modulating intracellular VEGF transport
- PMID: 11435456
- PMCID: PMC209338
- DOI: 10.1172/JCI11772
Expression of the oxygen-regulated protein ORP150 accelerates wound healing by modulating intracellular VEGF transport
Abstract
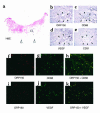
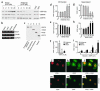
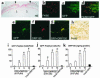
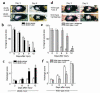
Expression of angiogenic factors such as VEGF under conditions of hypoxia or other kinds of cell stress contributes to neovascularization during wound healing. The inducible endoplasmic reticulum chaperone oxygen-regulated protein 150 (ORP150) is expressed in human wounds along with VEGF. Colocalization of these two molecules was observed in macrophages in the neovasculature, suggesting a role of ORP150 in the promotion of angiogenesis. Local administration of ORP150 sense adenovirus to wounds of diabetic mice, a treatment that efficiently targeted this gene product to the macrophages of wound beds, increased VEGF antigen in wounds and accelerated repair and neovascularization. In cultured human macrophages, inhibition of ORP150 expression caused retention of VEGF antigen within the endoplasmic reticulum (ER), while overexpression of ORP150 promoted the secretion of VEGF into hypoxic culture supernatants. Taken together, these data suggest an important role for ORP150 in the setting of impaired wound repair and identify a key, inducible chaperone-like molecule in the ER. This novel facet of the angiogenic response may be amenable to therapeutic manipulation.
Figures

Comment in
-
Regulation of hypoxia-induced angiogenesis: a chaperone escorts VEGF to the dance.J Clin Invest. 2001 Jul;108(1):39-40. doi: 10.1172/JCI13374. J Clin Invest. 2001. PMID: 11435455 Free PMC article. No abstract available.
References
-
- Ferrara N, et al. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. - PubMed
-
- Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. - PubMed
-
- Larcher F, et al. Up-regulation of vascular endothelial growth factor/vascular permeability factor in mouse skin carcinogenesis correlates with malignant progression state and activated H-ras expression levels. Cancer Res. 1996;56:5391–5396. - PubMed
-
- Machein MR, Risau W, Plate KH. Antiangiogenic gene therapy in a rat glioma model using a dominant-negative vascular endothelial growth factor receptor 2. Hum Gene Ther. 1999;10:1117–1128. - PubMed
-
- Alvarez Arroyo MV, et al. Role of vascular endothelial growth factor in the response to vessel injury. Kidney Int Suppl. 1998;68:S7–S9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

