A coordinated change in chemokine responsiveness guides plasma cell movements
- PMID: 11435471
- PMCID: PMC2193440
- DOI: 10.1084/jem.194.1.45
A coordinated change in chemokine responsiveness guides plasma cell movements
Abstract
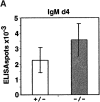
Antibody-secreting plasma cells are nonrecirculatory and lodge in splenic red pulp, lymph node medullary cords, and bone marrow. The factors that regulate plasma cell localization are poorly defined. Here we demonstrate that, compared with their B cell precursors, plasma cells exhibit increased chemotactic sensitivity to the CXCR4 ligand CXCL12. At the same time, they downregulate CXCR5 and CCR7 and have reduced responsiveness to the B and T zone chemokines CXCL13, CCL19, and CCL21. We demonstrate that CXCL12 is expressed within splenic red pulp and lymph node medullary cords as well as in bone marrow. In chimeric mice reconstituted with CXCR4-deficient fetal liver cells, plasma cells are mislocalized in the spleen, found in elevated numbers in blood, and fail to accumulate normally in the bone marrow. Our findings indicate that as B cells differentiate into plasma cells they undergo a coordinated change in chemokine responsiveness that regulates their movements in secondary lymphoid organs and promotes lodgment within the bone marrow.
Figures






References
-
- van Rooijen N., Claassen E., Eikelenboom P. Is there a single differentiation pathway for all antibody-forming cells in the spleen? Immunol. Today. 1986;7:193–196. - PubMed
-
- Liu Y.-J., Zhang J., Lane P.J.L., Chan E.Y.-T., MacLennan I.C.M. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur. J. Immunol. 1991;21:2951–2962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

