Brain mast cells act as an immune gate to the hypothalamic-pituitary-adrenal axis in dogs
- PMID: 11435473
- PMCID: PMC2193441
- DOI: 10.1084/jem.194.1.71
Brain mast cells act as an immune gate to the hypothalamic-pituitary-adrenal axis in dogs
Abstract
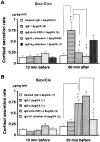
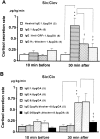
Mast cells perform a significant role in the host defense against parasitic and some bacterial infections. Here we show that in the dog, degranulation of brain mast cells evokes hypothalamic-pituitary-adrenal responses via histamine release. A large number of mast cells were found in a circumscribed ventral region of the hypothalamus, including the pars tuberalis and median eminence. When these intracranial mast cells were passively sensitized with immunoglobulin E via either the intracerebroventricular or intravenous route, there was a marked increase in the adrenal cortisol secretion elicited by a subsequent antigenic challenge (whether this was delivered via the central or peripheral route). Comp.48/80, a mast cell secretagogue, also increased cortisol secretion when administered intracerebroventricularly. Pretreatment (intracerebroventricularly) with anti-corticotropin--releasing factor antibodies or a histamine H(1) blocker, but not an H(2) blocker, attenuated the evoked increases in cortisol. These data show that in the dog, degranulation of brain mast cells evokes hypothalamic-pituitary-adrenal responses via centrally released histamine and corticotrophin-releasing factor. On the basis of these data, we suggest that intracranial mast cells may act as an allergen sensor, and that the activated adrenocortical response may represent a life-saving host defense reaction to a type I allergy.
Figures







Comment in
-
The diverse roles of mast cells.J Exp Med. 2001 Jul 2;194(1):F1-5. doi: 10.1084/jem.194.1.f1. J Exp Med. 2001. PMID: 11435478 Free PMC article. Review. No abstract available.
References
-
- Echtenacher D.N., Månnel D.N., Hültner L. Critical protective role of mast cells in a model of acute septic peritonitis. Nature. 1996;381:75–77. - PubMed
-
- Malaviya R., Ikeda T., Ross E., Abraham S.N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. - PubMed
-
- Chung K.F., Becher A.B., Lazarus S.C., Frick O.L., Dadel J.A., Gold W.M. Antigen-induced airway hyperresponsiveness and pulmonary inflammation in allergic dogs. J. Appl. Physiol. 1985;58:1347–1353. - PubMed
-
- Wagner E.M., Mitzner W.A., Bleecker E.R. Peripheral circulatory alterations in canine anaphylactic shock. Am. J. Physiol. 1986;251:H934–H940. - PubMed
-
- Edvinson L., Cervos-Narsson L.-I., Owman C.H., Ronnberg A.-L. Regional distribution of mast cells containing histamine, dopamine, or 5-hydroxytryptamine in the mammalian brain. Neurology. 1977;27:878–883. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

