Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro
- PMID: 11435587
- PMCID: PMC114435
- DOI: 10.1128/JVI.75.15.7067-7077.2001
Interferon-independent, human immunodeficiency virus type 1 gp120-mediated induction of CXCL10/IP-10 gene expression by astrocytes in vivo and in vitro
Abstract
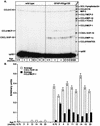
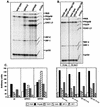
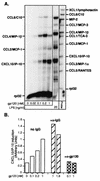
The CXC chemokine gamma interferon (IFN-gamma)-inducible protein CXCL10/IP-10 is markedly elevated in cerebrospinal fluid and brain of individuals infected with human immunodeficiency virus type 1 (HIV-1) and is implicated in the pathogenesis of HIV-associated dementia (HAD). To explore the possible role of CXCL10/IP-10 in HAD, we examined the expression of this and other chemokines in the central nervous system (CNS) of transgenic mice with astrocyte-targeted expression of HIV gp120 under the control of the glial fibrillary acidic protein (GFAP) promoter, a murine model for HIV-1 encephalopathy. Compared with wild-type controls, CNS expression of the CC chemokine gene CCL2/MCP-1 and the CXC chemokine genes CXCL10/IP-10 and CXCL9/Mig was induced in the GFAP-HIV gp120 mice. CXCL10/IP-10 RNA expression was increased most and overlapped the expression of the transgene-encoded HIV gp120 gene. Astrocytes and to a lesser extent microglia were identified as the major cellular sites for CXCL10/IP-10 gene expression. There was no detectable expression of any class of IFN or their responsive genes. In astrocyte cultures, soluble recombinant HIV gp120 protein was capable of directly inducing CXCL10/IP-10 gene expression a process that was independent of STAT1. These findings highlight a novel IFN- and STAT1-independent mechanism for the regulation of CXCL10/IP-10 expression and directly link expression of HIV gp120 to the induction of CXCL10/IP-10 that is found in HIV infection of the CNS. Finally, one function of IP-10 expression may be the recruitment of leukocytes to the CNS, since the brain of GFAP-HIV gp120 mice had increased numbers of CD3(+) T cells that were found in close proximity to sites of CXCL10/IP-10 RNA expression.
Figures





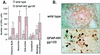
References
-
- Akwa Y, Hassett D E, Eloranta M L, Sandberg K, Masliah E, Powell H, Whitton J L, Bloom F E, Campbell I L. Transgenic expression of IFN-α in the central nervous system of mice protects against lethal neurotropic viral infection but induces inflammation and neurodegeneration. J Immunol. 1998;161:5016–5026. - PubMed
-
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
-
- Asensio V C, Campbell I L. Chemokines and their receptors in the CNS: directing cellular communication. Trends Neurosci. 1999;22:504–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

