Ethanol-induced apoptosis in mouse liver: Fas- and cytochrome c-mediated caspase-3 activation pathway
- PMID: 11438480
- PMCID: PMC1850406
- DOI: 10.1016/S0002-9440(10)61699-9
Ethanol-induced apoptosis in mouse liver: Fas- and cytochrome c-mediated caspase-3 activation pathway
Abstract
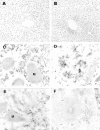
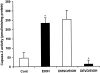
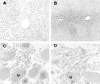
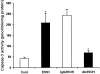
Hepatic apoptosis has been shown to occur in both experimental and clinical alcoholic liver disease, but the signaling pathway remains unknown. This study was undertaken to examine specifically the involvement of the upstream signals, Fas and cytochrome c, in alcohol-induced caspase-3 activation and apoptosis in the liver. Male FVB mice were administrated intragastrically a single dose of alcohol at 6 g/kg, which has been shown to represent binge drinking in humans. Hepatic apoptosis was detected by a terminal deoxynucleotidyl transferase dUTP nick-end labeling assay. Active form of caspase-3 was identified by immunoperoxidase staining and confirmed by immunogold labeling and was found to be in the cytosol and nucleus. Enzymic assay further confirmed caspase-3 activation and nucleus localization. Systemic administration of caspase-3 inhibitor, Ac-DEVD-FMK, inhibited caspase-3 activity and abrogated apoptosis. Elevation of cytosolic cytochrome c was found by immunoperoxidase staining, immunogold labeling, and Western blot. Increased Fas ligand expression was detected by immunoperoxidase staining. Intravenous administration of a neutralizing Fas ligand monoclonal antibody resulted in suppression of caspase-3 activation and attenuation of apoptosis, but did not inhibit mitochondrial cytochrome c release. The results thus demonstrate that Fas/Fas ligand system-mediated caspase-3 activation plays a central role in the ethanol-induced hepatic apoptosis.
Figures


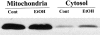


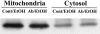
References
-
- Nanji AA: Apoptosis and alcoholic liver disease. Semin Liver Dis 1998, 18:187-190 - PubMed
-
- Slomiany BL, Piotrowski J, Piotrowski E, Slomiany A: Induction of buccal mucosal apoptosis with chronic alcohol ingestion. Biochem Mol Biol Int 1998, 44:381-389 - PubMed
-
- Slomiany BL, Piotrowski J, Slomiany A: Chronic alcohol ingestion enhances tumor necrosis factor-alpha expression and salivary gland apoptosis. Alcohol Clin Exp Res 1997, 21:1530-1533 - PubMed
-
- Piotrowski J, Piotrowski E, Slomiany A, Slomiany BL: Gastric mucosal apoptosis induced by ethanol: effect of antiulcer agents. Biochem Mol Biol Int 1997, 42:247-254 - PubMed
-
- Zhang FX, Rubin R, Rooney TA: Ethanol induces apoptosis in cerebellar granule neurons by inhibiting insulin-like growth factor 1 signaling. J Neurochem 1998, 71:196-204 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

