Stages of synapse development defined by dependence on F-actin
- PMID: 11438592
- PMCID: PMC6762826
- DOI: 10.1523/JNEUROSCI.21-14-05169.2001
Stages of synapse development defined by dependence on F-actin
Erratum in
- J Neurosci 2001 Sep 1;21(17):6991
Abstract
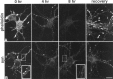
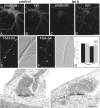
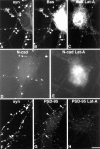
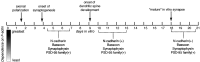
It has been widely speculated that actin plays a central role in CNS synapse assembly, but such a requirement for actin filaments (F-actin) has not yet been demonstrated experimentally. We used hippocampal neurons grown in culture and the actin depolymerizing agent, latrunculin A, to examine directly the relationship between F-actin and synapse formation and maturation. During the first week in culture, actin depolymerization results in a near complete loss of synapses defined by synaptophysin-labeled vesicle clusters, synaptic vesicle recycling, and ultrastructure. Over the second week in culture, F-actin becomes increasingly stable, but actin depolymerization no longer disrupts basic synaptic structure. There is, however, a reduction in the number and size of synaptophysin-labeled clusters and in the size of vesicle clusters undergoing FM4-64 recycling, suggesting that synaptic vesicle anchoring remains partially dependent on F-actin. By 18 d in culture, synaptophysin clusters and synaptic vesicle recycling are largely resistant to F-actin depolymerization. The decrease in synapse dependence on F-actin correlates well with the acquisition and retention of presynaptic scaffolding proteins such as Bassoon and postsynaptic scaffolding proteins such as those of the postsynaptic density-95 family. Increased activity stabilizes F-actin and its associated proteins at synaptic sites, suggesting a correlation between active synapses, actin stability, and synapse stability. Our findings demonstrate that F-actin is essential for the development and maintenance of young synapses. Because F-actin is also highly regulatable, we propose that F-actin may be a principal target for stabilizing or destabilizing signals that ultimately result in synapse maintenance or elimination.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
