Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats
- PMID: 11438612
- PMCID: PMC6762859
- DOI: 10.1523/JNEUROSCI.21-14-05358.2001
Local injection of endothelin-1 produces pain-like behavior and excitation of nociceptors in rats
Abstract
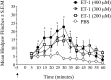
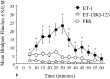
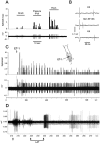
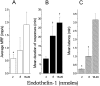
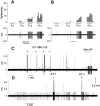
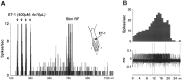
Neurobehavioral and neurophysiological actions of the peptide endothelin-1 (ET-1) were investigated after subcutaneous plantar hindpaw injections in adult male Sprague Dawley rats. Hindpaw flinching developed within minutes after ET-1 (8-16 nmol) injection, peaked at 30 min, lasted for 60 min, and was strongly inhibited by the endothelin-A (ET(A)) receptor antagonist, BQ-123 (3.2 m). In separate experiments, impulse activity of single, physiologically characterized sensory C-, Adelta-, and Abeta-fibers was recorded from the sciatic nerve in anesthetized rats after subcutaneous injections of endothelin-1 (1-20 nmol), alone or together with BQ-123 (3.2 m), into the plantar hindpaw receptive fields of these units. All nociceptive C-fibers (31 of 33 C-fibers studied) were excited by ET-1 (1-20 nmol) in a dose-dependent manner. For doses of 16-20 nmol, the mean latency for afferent activation after injection of ET-1 was 3.16 +/- 0.31 min, and the mean and maximum response frequency were 2.02 +/- 0.48 impulses (imp)/sec and 14.0 +/- 3.2 imp/sec, respectively. All 10 nociceptive Adelta-fibers (of 12 Adelta-fibers studied) also responded to 1-20 nmol of ET-1 in a dose-dependent manner with a mean latency of 3.5 +/- 0.12 min and mean response frequency of 3.3 +/- 2.3 imp/sec. In contrast, most Abeta-fibers (9 of 12) did not respond to ET-1. BQ-123, when coinjected with ET-1, blocked ET-1-induced activation in all C- and Adelta-fibers tested. These data demonstrate that subcutaneous administration of ET-1 to the rat plantar hindpaw produces pain-like behavior and selective excitation of nociceptive fibers through activation of ET(A) receptors.
Figures

References
-
- Carducci MA, Bowling MK, Rogers T, Leahy TL, Janus TJ, Padley RJ, Nelson JB (1998) Endothelin receptor antagonist, ABT-627, for prostate cancer: initial trial results. Presented at American Association for Cancer Research Meeting, Indian Wells, CA, December. Abstr. C-24.
-
- Crossman DC, Brain SD, Fuller RW. Potent vasoactive properties of endothelin 1 in human skin. J Appl Physiol. 1991;70:260–266. - PubMed
-
- Dahlof B, Gustafsson D, Hedner T, Jern S, Hannsson L. Regional hemodynamic effects of endothelin-1 in rat and man: unexpected adverse effects. J Hypertens. 1990;8:811–818. - PubMed
-
- Davar G, Hans G, Fareed MU, Sinnott C, Strichartz G. Behavioral signs of acute pain produced by application of endothelin-1 to rat sciatic nerve. NeuroReport. 1998;9:2279–2283. - PubMed
-
- De-Melo JD, Tonussi CR, D'Orleans-Juste P, Rae GA. Articular nociception induced by endothelin-1, carrageenan and LPS in naive and previously inflamed knee-joints in the rat: inhibition by endothelin receptor antagonists. Pain. 1998;77:261–269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
