G(alpha)11 signaling through ARF6 regulates F-actin mobilization and GLUT4 glucose transporter translocation to the plasma membrane
- PMID: 11438680
- PMCID: PMC87250
- DOI: 10.1128/MCB.21.15.5262-5275.2001
G(alpha)11 signaling through ARF6 regulates F-actin mobilization and GLUT4 glucose transporter translocation to the plasma membrane
Abstract
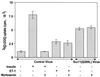
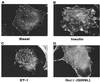
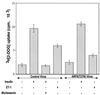
The action of insulin to recruit the intracellular GLUT4 glucose transporter to the plasma membrane of 3T3-L1 adipocytes is mimicked by endothelin 1, which signals through trimeric G(alpha)q or G(alpha)11 proteins. Here we report that murine G(alpha)11 is most abundant in fat and that expression of the constitutively active form of G(alpha)11 [G(alpha)11(Q209L)] in 3T3-L1 adipocytes causes recruitment of GLUT4 to the plasma membrane and stimulation of 2-deoxyglucose uptake. In contrast to the action of insulin on GLUT4, the effects of endothelin 1 and G(alpha)11 were not inhibited by the phosphatidylinositol 3-kinase inhibitor wortmannin at 100 nM. Signaling by insulin, endothelin 1, or G(alpha)11(Q209L) also mobilized cortical F-actin in cultured adipocytes. Importantly, GLUT4 translocation caused by all three agents was blocked upon disassembly of F-actin by latrunculin B, suggesting that the F-actin polymerization caused by these agents may be required for their effects on GLUT4. Remarkably, expression of a dominant inhibitory form of the actin-regulatory GTPase ARF6 [ARF6(T27N)] in cultured adipocytes selectively inhibited both F-actin formation and GLUT4 translocation in response to endothelin 1 but not insulin. These data indicate that ARF6 is a required downstream element in endothelin 1 signaling through G(alpha)11 to regulate cortical actin and GLUT4 translocation in cultured adipocytes, while insulin action involves different signaling pathways.
Figures








References
-
- Baldini G, Hohman R, Charron M J, Lodish H F. Insulin and nonhydrolyzable GTP analogs induce translocation of GLUT 4 to the plasma membrane in alpha-toxin-permeabilized rat adipose cells. J Biol Chem. 1991;266:4037–4040. - PubMed
-
- Baumann C A, Ribon V, Kanzaki M, Thurmond D C, Mora S, Shigematsu S, Bickel P E, Pessin J E, Saltiel A R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. - PubMed
-
- Berridge M J. Inositol trisphosphate and calcium signalling. Nature. 1993;361:315–325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
