Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation
- PMID: 11438690
- PMCID: PMC37474
- DOI: 10.1073/pnas.141230798
Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation
Abstract
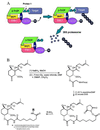
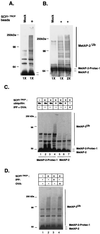
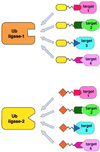
The intracellular levels of many proteins are regulated by ubiquitin-dependent proteolysis. One of the best-characterized enzymes that catalyzes the attachment of ubiquitin to proteins is a ubiquitin ligase complex, Skp1-Cullin-F box complex containing Hrt1 (SCF). We sought to artificially target a protein to the SCF complex for ubiquitination and degradation. To this end, we tested methionine aminopeptidase-2 (MetAP-2), which covalently binds the angiogenesis inhibitor ovalicin. A chimeric compound, protein-targeting chimeric molecule 1 (Protac-1), was synthesized to recruit MetAP-2 to SCF. One domain of Protac-1 contains the I kappa B alpha phosphopeptide that is recognized by the F-box protein beta-TRCP, whereas the other domain is composed of ovalicin. We show that MetAP-2 can be tethered to SCF(beta-TRCP), ubiquitinated, and degraded in a Protac-1-dependent manner. In the future, this approach may be useful for conditional inactivation of proteins, and for targeting disease-causing proteins for destruction.
Figures


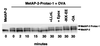
References
-
- Ciechanover A, Orian A, Schwartz A L. BioEssays. 2000;22:442–451. - PubMed
-
- Winston J F, Koepp D M, Zhu C, Elledge S J, Harper J W. Curr Biol. 1999;9:1180–1182. - PubMed
-
- Deshaies R J. Annu Rev Cell Dev Biol. 1999;15:435–467. - PubMed
-
- Yaron A, Hatzubai A, Davis M, Lagoon I, Amit S, Manning A M, Andersen J S, Mann M, Mercurio F, Ben-Neriah Y. Nature (London) 1998;396:590–594. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

