Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development
- PMID: 11438701
- PMCID: PMC37490
- DOI: 10.1073/pnas.151236498
Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development
Abstract
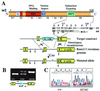
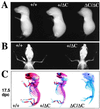
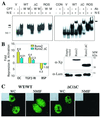
Runx (Cbfa/AML) transcription factors are critical for tissue-specific gene expression. A unique targeting signal in the C terminus directs Runx factors to discrete foci within the nucleus. Using Runx2/CBFA1/AML3 and its essential role in osteogenesis as a model, we investigated the fundamental importance of fidelity of subnuclear localization for tissue differentiating activity by deleting the intranuclear targeting signal via homologous recombination. Mice homozygous for the deletion (Runx2 Delta C) do not form bone due to maturational arrest of osteoblasts. Heterozygotes do not develop clavicles, but are otherwise normal. These phenotypes are indistinguishable from those of the homozygous and heterozygous null mutants, indicating that the intranuclear targeting signal is a critical determinant for function. The expressed truncated Runx2 Delta C protein enters the nucleus and retains normal DNA binding activity, but shows complete loss of intranuclear targeting. These results demonstrate that the multifunctional N-terminal region of the Runx2 protein is not sufficient for biological activity. We conclude that subnuclear localization of Runx factors in specific foci together with associated regulatory functions is essential for control of Runx-dependent genes involved in tissue differentiation during embryonic development.
Figures


References
-
- van Steensel B, Jenster G, Damm K, Brinkmann A O, van Driel R. J Cell Biochem. 1995;57:465–478. - PubMed
-
- Lamond A I, Earnshaw W C. Science. 1998;280:547–553. - PubMed
-
- Tang Y, Getzenberg R H, Vietmeier B N, Stallcup M R, Eggert M, Renkawitz R, DeFranco D B. Mol Endocrinol. 1998;12:1420–1431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

