Folliculostellate cell network: a route for long-distance communication in the anterior pituitary
- PMID: 11438713
- PMCID: PMC37531
- DOI: 10.1073/pnas.151339598
Folliculostellate cell network: a route for long-distance communication in the anterior pituitary
Abstract
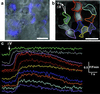
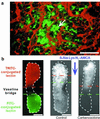
All higher life forms critically depend on hormones being rhythmically released by the anterior pituitary. The proper functioning of this master gland is dynamically controlled by a complex set of regulatory mechanisms that ultimately determine the fine tuning of the excitable endocrine cells, all of them heterogeneously distributed throughout the gland. Here, we provide evidence for an intrapituitary communication system by which information is transferred via the network of nonendocrine folliculostellate (FS) cells. Local electrical stimulation of FS cells in acute pituitary slices triggered cytosolic calcium waves, which propagated to other FS cells by signaling through gap junctions. Calcium wave initiation was because of the membrane excitability of FS cells, hitherto classified as silent cells. FS cell coupling could relay information between opposite regions of the gland. Because FS cells respond to central and peripheral stimuli and dialogue with endocrine cells, the form of large-scale intrapituitary communication described here may provide an efficient mechanism that orchestrates anterior pituitary functioning in response to physiological needs.
Figures




References
-
- Knobil E. Recent Prog Horm Res. 1980;36:53–88. - PubMed
-
- Liu J H, Kazer R R, Rasmussen D D. J Clin Endocrinol Metab. 1987;64:1027–1035. - PubMed
-
- Sassin J F, Frantz A G, Weitzman E D, Kapen S. Science. 1972;177:1205–1207. - PubMed
-
- Plotsky P M, Vale W. Science. 1985;230:461–463. - PubMed
-
- Porter J C, Ondo J G, Cramer O M. Handb Physiol Sect Endocrinol. 1974;4:33–43.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

