Methionine regeneration and aspartate aminotransferase in parasitic protozoa
- PMID: 11443076
- PMCID: PMC95336
- DOI: 10.1128/JB.183.15.4421-4434.2001
Methionine regeneration and aspartate aminotransferase in parasitic protozoa
Abstract
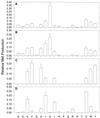
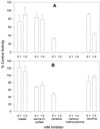
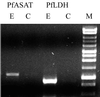
Aspartate aminotransferases have been cloned and expressed from Crithidia fasciculata, Trypanosoma brucei brucei, Giardia intestinalis, and Plasmodium falciparum and have been found to play a role in the final step of methionine regeneration from methylthioadenosine. All five enzymes contain sequence motifs consistent with membership in the Ia subfamily of aminotransferases; the crithidial and giardial enzymes and one trypanosomal enzyme were identified as cytoplasmic aspartate aminotransferases, and the second trypanosomal enzyme was identified as a mitochondrial aspartate aminotransferase. The plasmodial enzyme contained unique sequence substitutions and appears to be highly divergent from the existing members of the Ia subfamily. In addition, the P. falciparum enzyme is the first aminotransferase found to lack the invariant residue G197 (P. K. Mehta, T. I. Hale, and P. Christen, Eur. J. Biochem. 214:549-561, 1993), a feature shared by sequences discovered in P. vivax and P. berghei. All five enzymes were able to catalyze aspartate-ketoglutarate, tyrosine-ketoglutarate, and amino acid-ketomethiobutyrate aminotransfer reactions. In the latter, glutamate, phenylalanine, tyrosine, tryptophan, and histidine were all found to be effective amino donors. The crithidial and trypanosomal cytosolic aminotransferases were also able to catalyze alanine-ketoglutarate and glutamine-ketoglutarate aminotransfer reactions and, in common with the giardial aminotransferase, were able to catalyze the leucine-ketomethiobutyrate aminotransfer reaction. In all cases, the kinetic constants were broadly similar, with the exception of that of the plasmodial enzyme, which catalyzed the transamination of ketomethiobutyrate significantly more slowly than aspartate-ketoglutarate aminotransfer. This result obtained with the recombinant P. falciparum aminotransferase parallels the results seen for total ketomethiobutyrate transamination in malarial homogenates; activity in the latter was much lower than that in homogenates from other organisms. Total ketomethiobutyrate transamination in Trichomonas vaginalis and G. intestinalis homogenates was extensive and involved lysine-ketomethiobutyrate enzyme activity in addition to the aspartate aminotransferase activity. The methionine production in these two species could be inhibited by the amino-oxy compounds canaline and carboxymethoxylamine. Canaline was also found to be an uncompetitive inhibitor of the plasmodial aspartate aminotransferase, with a K(i) of 27 microm.
Figures




References
-
- Ayala F J, Escalante A A, Lal A A, Rich S M. Evolutionary relationships of human malaria parasites. In: Sherman I, editor. Malaria: parasite biology, pathogenesis, and protection. Washington, D.C.: ASM Press; 1998. pp. 285–300.
-
- Backlund P S, Chang C P, Smith R A. Identification of 2-keto-4-methylthiobutyrate as an intermediate compound in methionine synthesis from 5′-methylthioadenosine. J Biol Chem. 1982;257:4196–4202. - PubMed
-
- Backlund P S, Smith R A. Methionine synthesis from 5′-methylthioadenosine in rat liver. J Biol Chem. 1980;256:1533–1535. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

