Eukaryotic translation initiation factor 4E-dependent translation is not essential for survival of starved yeast cells
- PMID: 11443081
- PMCID: PMC95341
- DOI: 10.1128/JB.183.15.4477-4483.2001
Eukaryotic translation initiation factor 4E-dependent translation is not essential for survival of starved yeast cells
Abstract
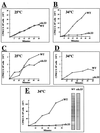
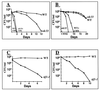
The eukaryotic translation initiation factor 4E (eIF4E) interacts with the mRNA 5' cap structure (m(7)GpppX) and is essential for the appropriate translation of the vast majority of eukaryotic mRNAs. Most studies of the yeast Saccharomyces cerevisiae CDC33 gene product, eIF4E, have been carried out with logarithmically growing cells, and little is known about its role in starved, nonproliferating cells that enter the stationary phase (SP). It has previously been found that the rate of translation in SP cells is more than 2 orders of magnitude lower than it is in dividing yeast cells. Here we show that this low rate of translation is essential for maintaining the viability of starved yeast cells that enter SP. Specifically, starved cells whose eIF4A is inactive or treated with cycloheximide rapidly lose viability. Moreover, after heat inactivation of the cdc33 temperature-sensitive product, the synthesis of most proteins is abolished and only a small group of proteins is still produced. Unexpectedly, starved cdc33 mutant cells whose eIF4E is inactive and which therefore fail to synthesize the bulk of their proteins remain viable for long periods of time, indistinguishable from their isogenic wild-type counterparts. Taken together, our results indicate that eIF4E-independent translation is necessary and sufficient for survival of yeast cells during long periods of starvation.
Figures

References
-
- Banroques J, Delahodde A, Jacq C. A mitochondrial RNA maturase gene transferred to the yeast nucleus can control mitochondrial mRNA splicing. Cell. 1986;46:837–844. - PubMed
-
- Barnes C A, MacKenzie M M, Johnston G C, Singer R A. Efficient translation of an SSA1-derived heat-shock mRNA in yeast cells limited for cap-binding protein and eIF-4F. Mol Gen Genet. 1995;246:619–627. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

