Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival
- PMID: 11443085
- PMCID: PMC95345
- DOI: 10.1128/JB.183.15.4509-4516.2001
Respiration capacity of the fermenting bacterium Lactococcus lactis and its positive effects on growth and survival
Abstract
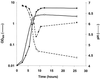
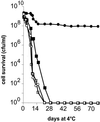
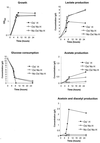
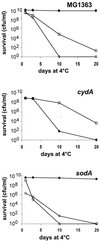
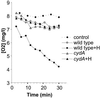
Oxygen is a major determinant of both survival and mortality of aerobic organisms. For the facultative anaerobe Lactococcus lactis, oxygen has negative effects on both growth and survival. We show here that oxygen can be beneficial to L. lactis if heme is present during aerated growth. The growth period is extended and long-term survival is markedly improved compared to results obtained under the usual fermentation conditions. We considered that improved growth and survival could be due to the capacity of L. lactis to undergo respiration. To test this idea, we confirmed that the metabolic behavior of lactococci in the presence of oxygen and hemin is consistent with respiration and is most pronounced late in growth. We then used a genetic approach to show the following. (i) The cydA gene, encoding cytochrome d oxidase, is required for respiration and plays a direct role in oxygen utilization. cydA expression is induced late in growth under respiration conditions. (ii) The hemZ gene, encoding ferrochelatase, which converts protoporphyrin IX to heme, is needed for respiration if the precursor, rather than the final heme product, is present in the medium. Surprisingly, survival improved by respiration is observed in a superoxide dismutase-deficient strain, a result which emphasizes the physiological differences between fermenting and respiring lactococci. These studies confirm respiratory metabolism in L. lactis and suggest that this organism may be better adapted to respiration than to traditional fermentative metabolism.
Figures
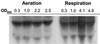
References
-
- Benov L, Fridovich I. A superoxide dismutase mimic protects sodA sodB Escherichia coli against aerobic heating and stationary-phase death. Arch Biochem Biophys. 1995;322:291–294. - PubMed
-
- Berlett B S, Stadtman E R. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. - PubMed
-
- Bolotin A, Mauger S, Malarme K, Ehrlich S D, Sorokin A. Low-redundancy sequencing of the entire Lactococcus lactis IL1403 genome. Antonie Leeuwenhoek. 1999;76:27–76. - PubMed
-
- Condon S. Responses of lactic acid bacteria to oxygen. FEMS Microbiol Rev. 1987;46:269–280.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

