The tumor-necrosis-factor receptor-associated periodic syndrome: new mutations in TNFRSF1A, ancestral origins, genotype-phenotype studies, and evidence for further genetic heterogeneity of periodic fevers
- PMID: 11443543
- PMCID: PMC1235304
- DOI: 10.1086/321976
The tumor-necrosis-factor receptor-associated periodic syndrome: new mutations in TNFRSF1A, ancestral origins, genotype-phenotype studies, and evidence for further genetic heterogeneity of periodic fevers
Erratum in
- Am J Hum Genet 2001 Nov;69(5):1160
Abstract
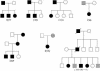
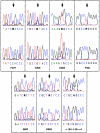
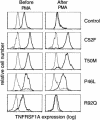
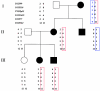
Mutations in the extracellular domain of the 55-kD tumor-necrosis factor (TNF) receptor (TNFRSF1A), a key regulator of inflammation, define a periodic-fever syndrome, TRAPS (TNF receptor-associated periodic syndrome [MIM 142680]), which is characterized by attacks of fever, sterile peritonitis, arthralgia, myalgia, skin rash, and/or conjunctivitis; some patients also develop systemic amyloidosis. Elsewhere we have described six disease-associated TNFRSF1A mutations, five of which disrupt extracellular cysteines involved in disulfide bonds; four other mutations have subsequently been reported. Among 150 additional patients with unexplained periodic fevers, we have identified four novel TNFRSF1A mutations (H22Y, C33G, S86P, and c.193-14 G-->A), one mutation (C30S) described by another group, and two substitutions (P46L and R92Q) present in approximately 1% of control chromosomes. The increased frequency of P46L and R92Q among patients with periodic fever, as well as functional studies of TNFRSF1A, argue that these are low-penetrance mutations rather than benign polymorphisms. The c.193-14 G-->A mutation creates a splice-acceptor site upstream of exon 3, resulting in a transcript encoding four additional extracellular amino acids. T50M and c.193-14 G-->A occur at CpG hotspots, and haplotype analysis is consistent with recurrent mutations at these sites. In contrast, although R92Q also arises at a CpG motif, we identified a common founder chromosome in unrelated individuals with this substitution. Genotype-phenotype studies identified, as carriers of cysteine mutations, 13 of 14 patients with TRAPS and amyloidosis and indicated a lower penetrance of TRAPS symptoms in individuals with noncysteine mutations. In two families with dominantly inherited disease and in 90 sporadic cases that presented with a compatible clinical history, we have not identified any TNFRSF1A mutation, despite comprehensive genomic sequencing of all of the exons, therefore suggesting further genetic heterogeneity of the periodic-fever syndromes.
Figures


References
Electronic-Database Information
-
- Centers for Disease Control and Prevention, http://www.cdc.gov/epiinfo/ei6.htm (for Epi Info 6 statistical software packages)
-
- HGSBASE, http://hgbase.cgr.ki.se (for allele frequencies of SNP in exon 1 [accession number SNP000005194] and SNP in intron 5 [accession number SNP000005195])
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim (for FCU [MIM 120100], FHF [MIM 142680], FMF [MIM 249100], HIDS [MIM 260920], and MWS [MIM 191900])
References
-
- Aganna E, Aksentijevich I, Hitman GA, Kastner DL, Hoepelman AIM, Posma FD, Zweers EJK, McDermott MF (2001) Tumor necrosis factor receptor-associated periodic fever syndrome (TRAPS) in a Dutch family: evidence for a TNFRSF1A mutation with reduced penetrance. Eur J Hum Genet 9:63–66 - PubMed
-
- Banner DW, D'Arcy A, Janes W, Gentz R, Schoenfeld H-J, Broger C, Loetscher H, Lesslauer W (1993) Crystal structure of the soluble human 55 kd TNF receptor-human TNFb complex: implications for TNF receptor activation. Cell 73:431–445 - PubMed
-
- Bazzoni F, Gatto L, Lenzi L, Vinante F, Pizzolo G, Zanolin E, DeGironcoli M (2000) Identification of novel polymorphisms in the human TNFR1 gene: distribution in acute leukemia patients and healthy individuals. Immunogenetics 51:159–163 - PubMed
-
- Centola M, Aksentijevich I, Kastner DL (1998) The hereditary periodic fever syndromes: molecular analysis of a new family of inflammatory diseases. Hum Mol Genet 7:1581–1588 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

