Activation of A(3)adenosine receptor protects against doxorubicin-induced cardiotoxicity
- PMID: 11444927
- PMCID: PMC10792614
- DOI: 10.1006/jmcc.2001.1387
Activation of A(3)adenosine receptor protects against doxorubicin-induced cardiotoxicity
Abstract
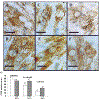
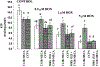
Adenosine exerts a marked protective effect on the heart during cardiac ischemia. This protection is mediated by binding to the A(1)and A(3)subtypes of adenosine receptor (A(1)R and A(3)R, respectively). The objective of the present study was to investigate whether activation of A(1)and A(3)adenosine receptors may reduce doxorubicin-induced damage to cardiomyocytes in culture. Cultured cardiomyocytes from newborn rats were treated with 0.5--5 microm doxorubicin (DOX) for 18 h and then incubated in drug-free medium for an additional 24 h. This treatment resulted in cell damage and lactate dehydrogenase release, even after low (0.5 microm) doses of the drug, and increased in a concentration-dependent manner. Activation of A(3)-subtype but not A(1)-subtype receptors attenuated doxorubicin-cardiotoxicity after drug treatment for 18 h followed by 24 h incubation in drug-free medium. Modulation of intracellular calcium mediated by activation of A(3)R, but not by A(1)R, in cultured myocytes suggested an important pathophysiological significance of this subtype of adenosine receptors. Protection by A(3)R agonist Cl-IB-MECA (2-chloro-N(6)-(3-iodobenzyl)adenosine-5'-N-methyluronamide) following DOX treatment is evident in: (1) decreases in intracellular calcium overloading and abnormalities in Ca(2+)transients; (2) reduction of free-radical generation and lipid peroxidation; (3) attenuation of mitochondrial damage by protection of the terminal link (COX-complex) of respiratory chain; (4) attenuation of the decrease in ATP production and irreversible cardiomyocyte damage. Cardioprotection caused by Cl-IB-MECA was antagonized considerably by the selective A(3)adenosine receptor antagonist MRS1523.
Copyright 2001 Academic Press.
Figures
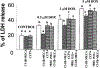


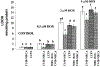
Similar articles
-
Cardioprotective effects of adenosine A1 and A3 receptor activation during hypoxia in isolated rat cardiac myocytes.Mol Cell Biochem. 2001 Jan;217(1-2):143-52. doi: 10.1023/a:1007209321969. Mol Cell Biochem. 2001. PMID: 11269659 Free PMC article.
-
Cardiomyocyte resistance to doxorubicin mediated by A(3) adenosine receptor.J Mol Cell Cardiol. 2002 May;34(5):493-507. doi: 10.1006/jmcc.2002.1532. J Mol Cell Cardiol. 2002. PMID: 12056854
-
Induction of apoptosis in rat cardiocytes by A3 adenosine receptor activation and its suppression by isoproterenol.Exp Cell Res. 2000 May 25;257(1):111-26. doi: 10.1006/excr.2000.4882. Exp Cell Res. 2000. PMID: 10854059 Free PMC article.
-
Adenosine A3 receptor-mediated cardioprotection against doxorubicin-induced mitochondrial damage.Biochem Pharmacol. 2010 Jan 15;79(2):180-7. doi: 10.1016/j.bcp.2009.08.010. Epub 2009 Aug 15. Biochem Pharmacol. 2010. PMID: 19686702
-
Purinergic signalling in brain ischemia.Neuropharmacology. 2016 May;104:105-30. doi: 10.1016/j.neuropharm.2015.11.007. Epub 2015 Nov 12. Neuropharmacology. 2016. PMID: 26581499 Review.
Cited by
-
TVP1022 protects neonatal rat ventricular myocytes against doxorubicin-induced functional derangements.J Pharmacol Exp Ther. 2010 Feb;332(2):413-20. doi: 10.1124/jpet.109.161158. Epub 2009 Nov 13. J Pharmacol Exp Ther. 2010. PMID: 19915070 Free PMC article.
-
Action of nucleosides and nucleotides at 7 transmembrane-spanning receptors.Nucleosides Nucleotides Nucleic Acids. 2006;25(12):1425-36. doi: 10.1080/15257770600919027. Nucleosides Nucleotides Nucleic Acids. 2006. PMID: 17067963 Free PMC article. Review.
-
Adenosine A3 receptors: novel ligands and paradoxical effects.Trends Pharmacol Sci. 1998 May;19(5):184-91. doi: 10.1016/s0165-6147(98)01203-6. Trends Pharmacol Sci. 1998. PMID: 9652191 Free PMC article. Review.
-
Involvement of UTP in protection of cardiomyocytes from hypoxic stress.Can J Physiol Pharmacol. 2009 Apr;87(4):287-99. doi: 10.1139/Y09-010. Can J Physiol Pharmacol. 2009. PMID: 19370082 Free PMC article.
-
Targeting GPCRs Against Cardiotoxicity Induced by Anticancer Treatments.Front Cardiovasc Med. 2020 Jan 24;6:194. doi: 10.3389/fcvm.2019.00194. eCollection 2019. Front Cardiovasc Med. 2020. PMID: 32039239 Free PMC article. Review.
References
-
- Mubagwa K, Mullane K, Flameng W. Role of adenosine in the heart circulation. Cardiovasc Res 1996; 32: 797–813. - PubMed
-
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998; 50: 413–492. - PubMed
-
- Auchampach JA, Bolli R. Adenosine receptor subtypes in the heart: therapeutic opportunities and challenges. Am J Physiol 1999; 276: H1113–H1116. - PubMed
-
- Tracey WR, Magee W, Masamune H, Kennedy SP, Knight DR, Buchholz RA, Hill RJ. Selective adenosine A3 receptor stimulation reduces ischemic myocardial injury in the rabbit heart. Cardiovasc Res 1997; 33: 410–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

