Nuclear reorganization and homologous chromosome pairing during meiotic prophase require C. elegans chk-2
- PMID: 11445542
- PMCID: PMC312723
- DOI: 10.1101/gad.902601
Nuclear reorganization and homologous chromosome pairing during meiotic prophase require C. elegans chk-2
Abstract
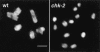
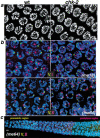
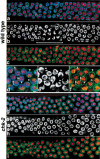
Analysis of mutants defective in meiotic chromosome pairing has uncovered a role for Caenorhabditis elegans chk-2 in initial establishment of pairing between homologous chromosomes during early meiotic prophase. chk-2 is also required for the major spatial reorganization of nuclei that normally accompanies the onset of pairing, suggesting a mechanistic coupling of these two events. Despite failures in pairing, nuclear reorganization, and crossover recombination, chk-2 mutants undergo many other aspects of meiotic chromosome morphogenesis and complete gametogenesis. Although chk-2 encodes a C. elegans ortholog of the Cds1/Chk2 checkpoint protein kinases, germ-line nuclei in chk-2 mutants are competent to arrest proliferation in response to replication inhibition and to trigger DNA damage checkpoint responses to ionizing radiation. However, chk-2 mutants are defective in triggering the pachytene DNA damage checkpoint in response to an intermediate block in the meiotic recombination pathway, suggesting that chk-2 is required either for initiation of meiotic recombination or for monitoring a specific subset of DNA damage lesions. We propose that chk-2 functions during premeiotic S phase to enable chromosomes to become competent for subsequent meiotic prophase events and/or to coordinate replication with entry into prophase.
Figures
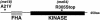

References
-
- Albertson DG, Rose AM, Villeneuve AM. Chromsome organization, mitosis, and meiosis. In: Riddle DL, Blumenthal T, Meyer BJ, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. pp. 47–78. - PubMed
-
- Bailis JM, Roeder GS. Pachytene exit controlled by reversal of Mek1-dependent phosphorylation. Cell. 2000;101:211–221. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
