Importin 13: a novel mediator of nuclear import and export
- PMID: 11447110
- PMCID: PMC125545
- DOI: 10.1093/emboj/20.14.3685
Importin 13: a novel mediator of nuclear import and export
Abstract
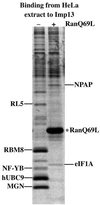
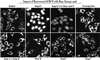
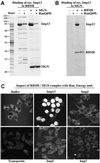
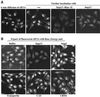
Importin beta-related receptors mediate translocation through nuclear pore complexes. Co-operation with the RanGTPase system allows them to bind and subsequently release their substrates on opposite sides of the nuclear envelope, which in turn ensures a directed nucleocytoplasmic transport. Here we identify a novel family member from higher eukaryotes that functions primarily, but not exclusively, in import. It accounts for nuclear accumulation of the SUMO-1/sentrin-conjugating enzyme hUBC9 and mediates import of the RBM8 (Y14) protein, and is therefore referred to as importin 13 (Imp13). Unexpectedly, Imp13 also shows export activity towards the translation initiation factor eIF1A and is thus a case where a single importin beta-like receptor transports different substrates in opposite directions. However, Imp13 operates differently from typical exportins in that the binding of eIF1A to Imp13 is only regulated indirectly by RanGTP, and the cytoplasmic release of eIF1A from Imp13 is triggered by the loading of import substrates onto Imp13.
Figures



Similar articles
-
Structural basis for the nuclear export activity of Importin13.EMBO J. 2013 Mar 20;32(6):899-913. doi: 10.1038/emboj.2013.29. Epub 2013 Feb 22. EMBO J. 2013. PMID: 23435562 Free PMC article.
-
Interactions between HIV Rev and nuclear import and export factors: the Rev nuclear localisation signal mediates specific binding to human importin-beta.J Mol Biol. 1997 Dec 19;274(5):693-707. doi: 10.1006/jmbi.1997.1420. J Mol Biol. 1997. PMID: 9405152
-
Exportin 4: a mediator of a novel nuclear export pathway in higher eukaryotes.EMBO J. 2000 Aug 15;19(16):4362-71. doi: 10.1093/emboj/19.16.4362. EMBO J. 2000. PMID: 10944119 Free PMC article.
-
Importin-beta-like nuclear transport receptors.Genome Biol. 2001;2(6):REVIEWS3008. doi: 10.1186/gb-2001-2-6-reviews3008. Epub 2001 Jun 5. Genome Biol. 2001. PMID: 11423015 Free PMC article. Review.
-
Distinct nuclear import and export pathways mediated by members of the karyopherin beta family.J Cell Biochem. 1998 Aug 1;70(2):231-9. J Cell Biochem. 1998. PMID: 9671229 Review.
Cited by
-
Pharmacogenomic Response of Inhaled Corticosteroids for the Treatment of Asthma: Considerations for Therapy.Pharmgenomics Pers Med. 2020 Aug 4;13:261-271. doi: 10.2147/PGPM.S231471. eCollection 2020. Pharmgenomics Pers Med. 2020. PMID: 32801837 Free PMC article. Review.
-
Partners and post-translational modifications of nuclear lamins.Chromosoma. 2013 Mar;122(1-2):13-31. doi: 10.1007/s00412-013-0399-8. Epub 2013 Mar 12. Chromosoma. 2013. PMID: 23475188 Free PMC article. Review.
-
Avermectin Derivatives, Pharmacokinetics, Therapeutic and Toxic Dosages, Mechanism of Action, and Their Biological Effects.Pharmaceuticals (Basel). 2020 Aug 17;13(8):196. doi: 10.3390/ph13080196. Pharmaceuticals (Basel). 2020. PMID: 32824399 Free PMC article. Review.
-
Self-organization of anastral spindles by synergy of dynamic instability, autocatalytic microtubule production, and a spatial signaling gradient.PLoS One. 2007 Feb 28;2(2):e244. doi: 10.1371/journal.pone.0000244. PLoS One. 2007. PMID: 17330139 Free PMC article.
-
Interaction between importin 13 and myopodin suggests a nuclear import pathway for myopodin.Mol Cell Biochem. 2008 Jan;307(1-2):93-100. doi: 10.1007/s11010-007-9588-1. Epub 2007 Sep 8. Mol Cell Biochem. 2008. PMID: 17828378
References
-
- Arts G.J., Fornerod,M. and Mattaj,I.W. (1998) Identification of a nuclear export receptor for tRNA. Curr. Biol., 8, 305–314. - PubMed
-
- Battiste J.L., Pestova,T.V., Hellen,C.U. and Wagner,G. (2000) The eIF1A solution structure reveals a large RNA-binding surface important for scanning function. Mol. Cell, 5, 109–119. - PubMed
-
- Benne R., Brown-Luedi,M.L. and Hershey,J.W. (1978) Purification and characterization of protein synthesis initiation factors eIF-1, eIF-4C, eIF-4D and eIF-5 from rabbit reticulocytes. J. Biol. Chem., 253, 3070–3077. - PubMed
-
- Bischoff F.R. and Görlich,D. (1997) RanBP1 is crucial for the release of RanGTP from importin β-related nuclear transport factors. FEBS Lett., 419, 249–254. - PubMed
-
- Bischoff F.R. and Ponstingl,H. (1991) Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature, 354, 80–82. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

