Positive and negative regulation of Raf kinase activity and function by phosphorylation
- PMID: 11447113
- PMCID: PMC125532
- DOI: 10.1093/emboj/20.14.3716
Positive and negative regulation of Raf kinase activity and function by phosphorylation
Abstract
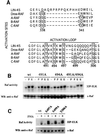
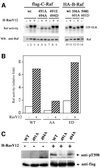
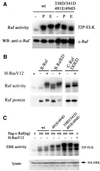
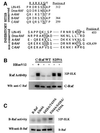
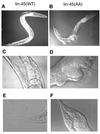
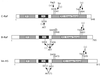
Activating and inhibitory phosphorylation mechanisms play an essential role in regulating Raf kinase activity. Here we demonstrate that phosphorylation of C-Raf in the kinase activation loop (residues T491 and S494) is necessary, but not sufficient, for activation. C-Raf has additional activating phosphorylation sites at S338 and Y341. Mutating all four of these residues to acidic residues, S338D/Y341D/T491E/S494D (DDED), in C-Raf results in constitutive activity. However, acidic residue substitutions at the corresponding activation loop sites in B-Raf are sufficient to confer constitutive activity. B-Raf and C-Raf also utilize similar inhibitory phosphorylation mechanisms to regulate kinase activity. B-Raf has multiple inhibitory phosphorylation sites necessary for full kinase inhibition where C-Raf requires only one. We examined the functional significance of these inhibitory and activating phosphorylations in Caenorhabditis elegans lin-45 Raf. Eliminating the inhibitory phosphorylation or mimicking activating phosphorylation sites is sufficient to confer constitutive activity upon lin-45 Raf and induce multi-vulva phenotypes in C.elegans. Our results demonstrate that different members of the Raf family kinases have both common and distinct phosphorylation mechanisms to regulate kinase activity and biological function.
Figures





References
-
- Abraham D. et al. (2000) Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J. Biol. Chem., 275, 22300–22304. - PubMed
-
- Barnard D., Diaz,B., Clawson,D. and Marshall,M. (1998) Oncogenes, growth factors and phorbol esters regulate Raf-1 through common mechanisms. Oncogene, 17, 1539–1547. - PubMed
-
- Beitel G.J., Clark,S.G. and Horvitz,H.R. (1990) Caenorhabditis elegans ras gene let-60 acts as a switch in the pathway of vulval induction. Nature, 348, 503–509. - PubMed
-
- Carroll M.P. and May,W.S. (1994) Protein kinase C-mediated serine phosphorylation directly activates Raf-1 in murine hematopoietic cells. J. Biol. Chem., 269, 1249–1256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

