Acf1, the largest subunit of CHRAC, regulates ISWI-induced nucleosome remodelling
- PMID: 11447119
- PMCID: PMC125259
- DOI: 10.1093/emboj/20.14.3781
Acf1, the largest subunit of CHRAC, regulates ISWI-induced nucleosome remodelling
Abstract
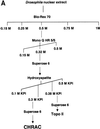
The chromatin accessibility complex (CHRAC) was originally defined biochemically as an ATP-dependent 'nucleosome remodelling' activity. Central to its activity is the ATPase ISWI, which catalyses the transfer of histone octamers between DNA segments in cis. In addition to ISWI, four other potential subunits were observed consistently in active CHRAC fractions. We have now identified the p175 subunit of CHRAC as Acf1, a protein known to associate with ISWI in the ACF complex. Interaction of Acf1 with ISWI enhances the efficiency of nucleosome sliding by an order of magnitude. Remarkably, it also modulates the nucleosome remodelling activity of ISWI qualitatively by altering the directionality of nucleosome movements and the histone 'tail' requirements of the reaction. The Acf1-ISWI heteromer tightly interacts with the two recently identified small histone fold proteins CHRAC-14 and CHRAC-16. Whether topoisomerase II is an integral subunit has been controversial. Refined analyses now suggest that topoisomerase II should not be considered a stable subunit of CHRAC. Accordingly, CHRAC can be molecularly defined as a complex consisting of ISWI, Acf1, CHRAC-14 and CHRAC-16.
Figures





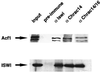






References
-
- Boyer L.A., Logie,C., Bonte,E., Becker,P.B., Wade,P.A., Wolffe,A.P., Wu,C., Imbalzano,A.N. and Peterson,C.L. (2000) Functional delineation of three groups of the ATP-dependent family of chromatin remodeling enzymes. J. Biol. Chem., 275, 18864–18870. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

