The RNA binding protein YB-1 binds A/C-rich exon enhancers and stimulates splicing of the CD44 alternative exon v4
- PMID: 11447123
- PMCID: PMC125550
- DOI: 10.1093/emboj/20.14.3821
The RNA binding protein YB-1 binds A/C-rich exon enhancers and stimulates splicing of the CD44 alternative exon v4
Abstract
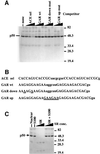
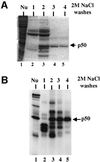
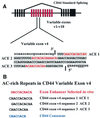
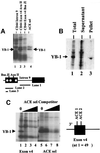
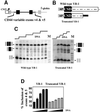
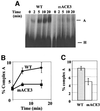
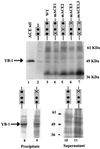
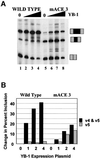
Exon enhancers are accessory pre-mRNA splicing signals that stimulate exon splicing. One class of proteins, the serine-arginine-rich (SR) proteins, have been demonstrated to bind enhancers and activate splicing. Here we report that A/C-rich exon enhancers (ACE elements) are recognized by the human YB-1 protein, a non-SR protein. Sequence-specific binding of YB-1 was observed both to an ACE derived from an in vivo iterative selection protocol and to ACE elements in an alternative exon (v4) from the human CD44 gene. The ACE element that was the predominant YB-1 binding site in CD44 exon v4 was required for maximal in vivo splicing and in vitro spliceosome assembly. Expression of wild-type YB-1 increased inclusion of exon v4, whereas a truncated form of YB-1 did not. Stimulation of exon v4 inclusion by wild-type YB-1 required the ACE necessary for YB-1 binding in vitro, suggesting that YB-1 stimulated exon inclusion in vivo by binding to an exonic ACE element. These observations identify a protein in addition to SR proteins that participates in the recognition of exon enhancers.
Figures



References
-
- Bargou R.C. et al. (1997) Nuclear localization and increased levels of transcription factor YB-1 in primary human breast cancers are associated with intrinsic MDR1 gene expression. Nature Med., 3, 447–478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

