Relationship of anti-Lewis x and anti-Lewis y antibodies in serum samples from gastric cancer and chronic gastritis patients to Helicobacter pylori-mediated autoimmunity
- PMID: 11447150
- PMCID: PMC98564
- DOI: 10.1128/IAI.69.8.4774-4781.2001
Relationship of anti-Lewis x and anti-Lewis y antibodies in serum samples from gastric cancer and chronic gastritis patients to Helicobacter pylori-mediated autoimmunity
Abstract
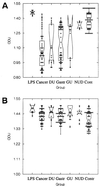
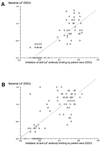
Lewis (Le) antigens have been implicated in the pathogenesis of atrophic gastritis and gastric cancer in the setting of Helicobacter pylori infection, and H. pylori-induced anti-Le antibodies have been described that cross-react with the gastric mucosa of both mice and humans. The aim of this study was to examine the presence of anti-Le antibodies in patients with H. pylori infection and gastric cancer and to examine the relationships between anti-Le antibody production, bacterial Le expression, gastric histopathology, and host Le erythrocyte phenotype. Anti-Le antibody production and H. pylori Le expression were determined by enzyme-linked immunosorbent assay, erythrocyte Le phenotype was examined by agglutination assays, and histology was scored blindly. Significant levels of anti-Le(x) antibody (P < 0.0001, T = 76.4, DF = 5) and anti-Le(y) antibody (P < 0.0001, T = 73.05, DF = 5) were found in the sera of patients with gastric cancer and other H. pylori-associated pathology compared with H. pylori-negative controls. Following incubation of patient sera with synthetic Le glycoconjugates, anti-Le(x) and -Le(y) autoantibody binding was abolished. The degree of the anti-Le(x) and -Le(y) antibody response was unrelated to the host Le phenotype but was significantly associated with the bacterial expression of Le(x) (r = 0.863, r(2) = 0.745, P < 0.0001) and Le(y) (r = 0.796, r(2) = 0.634, P < 0.0001), respectively. Collectively, these data suggest that anti-Le antibodies are present in most patients with H. pylori infection, including those with gastric cancer, that variability exists in the strength of the anti-Le response, and that this response is independent of the host Le phenotype but related to the bacterial Le phenotype.
Figures

References
-
- Appelmelk B J, Faller G, Claeys D, Kirchner T, Vandenbroucke-Grauls C M J E. Bugs on trial: the case of Helicobacter pylori and autoimmunity. Immunol Today. 1998;19:296–299. - PubMed
-
- Appelmelk B J, Monteiro M A, Martin S L, Moran A P, Vandenbroucke-Grauls C M J E. Why Heicobacter pylori has Lewis antigens. Trends Microbiol. 2000;8:565–570. - PubMed
-
- Appelmelk B J, Simoons-Smit I, Negrini R, Moran A P, Aspinall G O, Forte J G, de Vries T, Quan H, Verboom T, Maaskant J G, Ghiara P, Kuipers E J, Bloemena E, Tadema T M, Townsend R R, Tyagarajan K, Crothers J M, Jr, Montiero M A, Savio A, de Graaff J. Potential role of molecular mimicry between Helicobacter pylori lipopolysaccharide and host Lewis blood group antigens in autoimmunity. Infect Immun. 1996;64:2031–2040. - PMC - PubMed
-
- Aspinall G O, Monteiro M A. Lipopolysaccharides of Helicobacter pylori strains P466 and MO19: structures of the O antigen and core oligosaccharide regions. Biochemistry. 1996;35:2498–2504. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

