Immunogenicity of well-characterized synthetic Plasmodium falciparum multiple antigen peptide conjugates
- PMID: 11447164
- PMCID: PMC98578
- DOI: 10.1128/IAI.69.8.4884-4890.2001
Immunogenicity of well-characterized synthetic Plasmodium falciparum multiple antigen peptide conjugates
Abstract
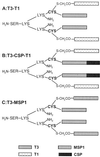
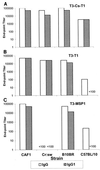
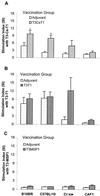
Given the emerging difficulties with malaria drug resistance and vector control, as well as the persistent lack of an effective vaccine, new malaria vaccine development strategies are needed. We used a novel methodology to synthesize and fully characterize multiple antigen peptide (MAP) conjugates containing protective epitopes from Plasmodium falciparum and evaluated their immunogenicity in four different strains of mice. A di-epitope MAP (T3-T1) containing two T-cell epitopes of liver stage antigen-1 (LSA-1), a di-epitope MAP containing T-cell epitopes from LSA-1 and from merozoite surface protein-1, and a tri-epitope MAP (T3-CS-T1) containing T3-T1 and a potent B-cell epitope from the circumsporozoite protein central repeat region were tested in this study. Mice of all four strains produced peptide-specific antibodies; however, the magnitude of the humoral response indicated strong genetic restriction between the different strains of mice. Anti-MAP antibodies recognized stage-specific proteins on the malaria parasites in an immunofluorescence assay. In addition, serum from hybrid BALB/cJ x A/J CAF1 mice that had been immunized with the tri-epitope MAP T3-CS-T1 successfully inhibited the malaria sporozoite invasion of hepatoma cells in vitro. Spleen cells from immunized mice also showed a genetically restricted cellular immune response when stimulated with the immunogen in vitro. This study indicates that well-characterized MAPs combining solid-phase synthesis and conjugation chemistries are potent immunogens and that this approach can be utilized for the development of subunit vaccines.
Figures
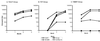
Similar articles
-
Binding of malaria T cell epitopes to DR and DQ molecules in vitro correlates with immunogenicity in vivo: identification of a universal T cell epitope in the Plasmodium falciparum circumsporozoite protein.J Immunol. 1997 Aug 1;159(3):1362-73. J Immunol. 1997. PMID: 9233633
-
Immunogenicity of multiple antigen peptides (MAP) containing T and B cell epitopes of the repeat region of the P. falciparum circumsporozoite protein.Eur J Immunol. 1991 Dec;21(12):3015-20. doi: 10.1002/eji.1830211217. Eur J Immunol. 1991. PMID: 1721025
-
Immunogenicity and in vitro protective efficacy of a polyepitope Plasmodium falciparum candidate vaccine constructed by epitope shuffling.Vaccine. 2007 Jul 9;25(28):5155-65. doi: 10.1016/j.vaccine.2007.04.085. Epub 2007 May 21. Vaccine. 2007. PMID: 17548134
-
T cell responses to repeat and non-repeat regions of the circumsporozoite protein detected in volunteers immunized with Plasmodium falciparum sporozoites.Mem Inst Oswaldo Cruz. 1992;87 Suppl 3:223-7. doi: 10.1590/s0074-02761992000700037. Mem Inst Oswaldo Cruz. 1992. PMID: 1364202 Review.
-
How to induce protective humoral immunity against Plasmodium falciparum circumsporozoite protein.J Exp Med. 2022 Feb 7;219(2):e20201313. doi: 10.1084/jem.20201313. Epub 2022 Jan 10. J Exp Med. 2022. PMID: 35006242 Free PMC article. Review.
Cited by
-
DNA prime and peptide boost immunization protocol encoding the Toxoplasma gondii GRA4 induces strong protective immunity in BALB/c mice.BMC Infect Dis. 2013 Oct 23;13:494. doi: 10.1186/1471-2334-13-494. BMC Infect Dis. 2013. PMID: 24148219 Free PMC article.
-
Identification and characterization of protective CD8+ T-epitopes in a malaria vaccine candidate SLTRiP.Immun Inflamm Dis. 2020 Mar;8(1):50-61. doi: 10.1002/iid3.283. Epub 2020 Jan 22. Immun Inflamm Dis. 2020. PMID: 31967737 Free PMC article.
-
Proteins of the Plasmodium falciparum two transmembrane Maurer's cleft protein family, PfMC-2TM, and the 130 kDa Maurer's cleft protein define different domains of the infected erythrocyte intramembranous network.Parasitol Res. 2009 Mar;104(4):875-91. doi: 10.1007/s00436-008-1270-3. Epub 2009 Jan 7. Parasitol Res. 2009. PMID: 19130087
-
A novel FGFR3-binding peptide inhibits FGFR3 signaling and reverses the lethal phenotype of mice mimicking human thanatophoric dysplasia.Hum Mol Genet. 2012 Dec 15;21(26):5443-55. doi: 10.1093/hmg/dds390. Epub 2012 Sep 26. Hum Mol Genet. 2012. PMID: 23014564 Free PMC article.
-
High throughput T epitope mapping and vaccine development.J Biomed Biotechnol. 2010;2010:325720. doi: 10.1155/2010/325720. Epub 2010 Jun 15. J Biomed Biotechnol. 2010. PMID: 20617148 Free PMC article. Review.
References
-
- Ahlborg N, Nardin E H, Perlmann P, Berzins K, Andersson R. Immunogenicity of chimeric multiple antigen peptides based on Plasmodium falciparum antigens: impact of epitope orientation. Vaccine. 1998;16:38–44. - PubMed
-
- Ballou W R, Hoffman S L, Sherwood J A, Hollingdale M R, Neva F A, Hockmeyer W T, Gordon D M, Schneider I, Wirtz R A, Young J F, et al. Safety and efficacy of a recombinant DNA Plasmodium falciparum sporozoite vaccine. Lancet. 1987;i:1277–1281. - PubMed
-
- Boykins R A, Joshi M, Syin C, Dhawan S, Nakhasi H. Synthesis and construction of a novel multiple peptide conjugate system: strategy for a subunit vaccine design. Peptides. 2000;21:9–17. - PubMed
-
- Brown G V. Progress in the development of malaria vaccines: context and constraints. Parassitologia. 1999;41:429–432. - PubMed
-
- Cerami C, Kwakye-Berko F, Nussenzweig V. Binding of malarial circumsporozoite protein to sulfatides [Gal(3-SO4)beta 1-Cer] and cholesterol-3-sulfate and its dependence on disulfide bond formation between cysteines in region II. Mol Biochem Parasitol. 1992;54:1–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

