Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology
- PMID: 11447166
- PMCID: PMC98580
- DOI: 10.1128/IAI.69.8.4898-4905.2001
Neutrophil depletion during Toxoplasma gondii infection leads to impaired immunity and lethal systemic pathology
Abstract
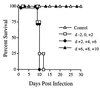
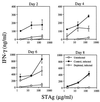
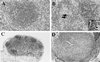
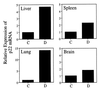
The immunomodulatory role of neutrophils during infection with Toxoplasma gondii was investigated. Monoclonal antibody-mediated depletion revealed that neutrophils are essential for survival during the first few days of infection. Moreover, neutrophil depletion was associated with a weaker type 1 immune response as measured by decreased levels of gamma interferon, interleukin-12 (IL-12) and tumor necrosis factor alpha. IL-10 was also decreased in depleted animals. Additionally, splenic populations of CD4(+) T cells, CD8(+) T cells, and NK1.1(+) cells were decreased in depleted mice. Neutrophil-depleted mice exhibited lesions of greater severity in tissues examined and a greater parasite burden as determined by histopathology and reverse transcription-PCR. We conclude that neutrophils are critical near the time of infection because they influence the character of the immune response and control tachyzoite replication.
Figures




References
-
- Alexander J, Hunter C A. Immunoregulation during toxoplasmosis. Chem Immunol. 1998;70:81–102. - PubMed
-
- Bliss S K, Butcher B A, Denkers E Y. Rapid recruitment of neutrophils with prestored IL-12 during microbial infection. J Immunol. 2000;165:4515–4521. - PubMed
-
- Bliss S K, Marshall A J, Zhang Y, Denkers E Y. Human polymorphonuclear leukocytes produce IL-12, TNF-α, and the chemokines macrophage-inflammatory protein-1 α and −1β in response to Toxoplasma gondii antigens. J Immunol. 1999;162:7369–7375. - PubMed
-
- Bliss S K, Zhang Y, Denkers E Y. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-γ-independent IL-12. J Immunol. 1999;163:2081–2088. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

