Meningococcal outer membrane vesicle vaccine given intranasally can induce immunological memory and booster responses without evidence of tolerance
- PMID: 11447180
- PMCID: PMC98594
- DOI: 10.1128/IAI.69.8.5010-5015.2001
Meningococcal outer membrane vesicle vaccine given intranasally can induce immunological memory and booster responses without evidence of tolerance
Abstract
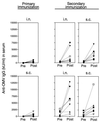
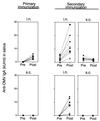
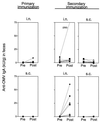
We have studied the ability of outer membrane vesicle (OMV) vaccines from Neisseria meningitidis serogroup B to induce vaccine-specific antibody and spleen cell proliferative responses in mice after being administered intranasally (i.n.) and/or subcutaneously (s.c.). A series of four weekly i.n. doses (25 microg) without adjuvant or a single s.c. dose (2.5 microg) with aluminum hydroxide was followed 2 months later by secondary i.n. or s.c. immunizations. After i.n. priming, both immunoglobulin G (IgG) antibody responses in serum, measured by enzyme-linked immunosorbent assay, and IgA antibodies in saliva and extracts of feces were significantly boosted by later i.n. immunizations. The IgG antibody responses in serum were also significantly augmented by secondary s.c. immunization after i.n. as well as s.c. priming. Sera from mice immunized i.n. reached the same level of bactericidal activity as after s.c. immunizations. The s.c. immunizations alone, however, had no effect on mucosal IgA antibody responses, but could prime for booster antibody responses in secretions to later i.n. immunizations. The i.n. immunizations also led to marked OMV-specific spleen cell proliferation in vitro. Both serum antibody responses and spleen cell proliferation were higher after i.n. priming and later s.c. immunizations than after s.c. immunizations alone. There was thus no evidence that i.n. priming had induced immunological tolerance within the B- or T-cell system. Our results indicate that a nonproliferating meningococcal OMV vaccine given i.n. can induce immunological memory and that it may be favorably combined with similar vaccines for injections.
Figures

Similar articles
-
Immunisation schedules for non-replicating nasal vaccines can be made simple by allowing time for development of immunological memory.Vaccine. 2004 Jun 2;22(17-18):2278-84. doi: 10.1016/j.vaccine.2003.11.040. Vaccine. 2004. PMID: 15149787
-
Effect of a booster dose of serogroup B meningococcal vaccine on antibody response to Neisseria meningitidis in mice vaccinated with different immunization schedules.FEMS Immunol Med Microbiol. 2005 Apr 1;44(1):35-42. doi: 10.1016/j.femsim.2004.11.013. FEMS Immunol Med Microbiol. 2005. PMID: 15780576
-
Antigen-specific T-cell responses in humans after intranasal immunization with a meningococcal serogroup B outer membrane vesicle vaccine.Infect Immun. 1999 Feb;67(2):921-7. doi: 10.1128/IAI.67.2.921-927.1999. Infect Immun. 1999. PMID: 9916109 Free PMC article.
-
Intranasal administration of a meningococcal outer membrane vesicle vaccine induces persistent local mucosal antibodies and serum antibodies with strong bactericidal activity in humans.Infect Immun. 1998 Apr;66(4):1334-41. doi: 10.1128/IAI.66.4.1334-1341.1998. Infect Immun. 1998. PMID: 9529050 Free PMC article.
-
Outer membrane vesicle: a macromolecule with multifunctional activity.Hum Vaccin Immunother. 2012 Jul;8(7):953-5. doi: 10.4161/hv.20166. Epub 2012 Jun 15. Hum Vaccin Immunother. 2012. PMID: 22699443 Review.
Cited by
-
Comparison of immune responses to gonococcal PorB delivered as outer membrane vesicles, recombinant protein, or Venezuelan equine encephalitis virus replicon particles.Infect Immun. 2005 Nov;73(11):7558-68. doi: 10.1128/IAI.73.11.7558-7568.2005. Infect Immun. 2005. PMID: 16239559 Free PMC article.
-
Exosomes in infectious diseases: insights into leishmaniasis pathogenesis, immune modulation, and therapeutic potential.Naunyn Schmiedebergs Arch Pharmacol. 2025 May;398(5):4913-4931. doi: 10.1007/s00210-024-03702-7. Epub 2024 Dec 19. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39702600 Review.
-
Intranasal vaccination with leishmanial antigens protects golden hamsters (Mesocricetus auratus) against Leishmania (Viannia) Braziliensis infection.PLoS Negl Trop Dis. 2015 Jan 8;9(1):e3439. doi: 10.1371/journal.pntd.0003439. eCollection 2015 Jan. PLoS Negl Trop Dis. 2015. PMID: 25569338 Free PMC article.
-
Isolation and Functions of Extracellular Vesicles Derived from Parasites: The Promise of a New Era in Immunotherapy, Vaccination, and Diagnosis.Int J Nanomedicine. 2020 Apr 28;15:2957-2969. doi: 10.2147/IJN.S250993. eCollection 2020. Int J Nanomedicine. 2020. PMID: 32425527 Free PMC article. Review.
-
Discovery of Salmonella virulence factors translocated via outer membrane vesicles to murine macrophages.Infect Immun. 2011 Jun;79(6):2182-92. doi: 10.1128/IAI.01277-10. Epub 2011 Apr 4. Infect Immun. 2011. PMID: 21464085 Free PMC article.
References
-
- Berstad A K H, Holst J, Møgster B, Haugen I L, Haneberg B. A nasal whole-cell pertussis vaccine can induce strong systemic and mucosal antibody responses which are not enhanced by cholera toxin. Vaccine. 1997;15:1473–1478. - PubMed
-
- Brandtzaeg P, Baklien K, Bjerke K, Rognum T O, Scott H, Valnes K. Nature and properties of the human gastrointestinal immune system. In: Miller K, Nicklin S, editors. Immunology of the gastrointestinal tract. Boca Raton, Fla: CRC Press, Inc.; 1987. pp. 1–85.
-
- Czerkinsky C, Holmgren J. The mucosal immune system and prospects for anti-infectious and anti-inflammatory vaccines. Immunologist. 1995;3:97–103.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

