Cell adhesion molecule and lymphocyte activation marker expression during experimental vaginal candidiasis
- PMID: 11447188
- PMCID: PMC98602
- DOI: 10.1128/IAI.69.8.5072-5079.2001
Cell adhesion molecule and lymphocyte activation marker expression during experimental vaginal candidiasis
Abstract
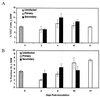
Cell-mediated immunity by Th1-type CD4(+) T cells is the predominant host defense mechanism against mucosal candidiasis. However, studies using an estrogen-dependent murine model of vaginal candidiasis have demonstrated little to no change in resident vaginal T cells during infection and no systemic T-cell infiltration despite the presence of Candida-specific systemic Th1-type responses in infected mice. The present study was designed to further investigate these observations by characterizing T-cell activation and cell adhesion molecule expression during primary and secondary C. albicans vaginal infections. While flow cytometry analysis of activation markers showed some evidence for activation of CD3(+) draining lymph node and/or vaginal lymphocytes during both primary and secondary vaginal Candida infection, CD3(+) cells expressing the homing receptors and integrins alpha(4)beta(7), alpha(M290)beta(7), and alpha(4)beta(1) in draining lymph nodes of mice with primary and secondary infections were reduced compared to results for uninfected mice. At the local level, few vaginal lymphocytes expressed integrins, with only minor changes observed during both primary and secondary infections. On the other hand, immunohistochemical analysis of vaginal cell adhesion molecule expression showed increases in mucosal addressin cell adhesion molecule 1 and vascular cell adhesion molecule 1 expression during both primary and secondary infections. Altogether, these data suggest that although the vaginal tissue is permissive to cellular infiltration during a vaginal Candida infection, the reduced numbers of systemic cells expressing the reciprocal cellular adhesion molecules may preempt cellular infiltration, thereby limiting Candida-specific T-cell responses against infection.
Figures


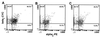

Similar articles
-
Differential expression of homing receptors and vascular addressins in tonsils and draining lymph nodes: Effect of Brucella infection in sheep.Vet Immunol Immunopathol. 2007 Feb 15;115(3-4):239-50. doi: 10.1016/j.vetimm.2006.11.008. Epub 2006 Nov 17. Vet Immunol Immunopathol. 2007. PMID: 17161868
-
Differential expression of homing molecules on recirculating lymphocytes from sheep gut, peripheral, and lung lymph.J Immunol. 1996 May 1;156(9):3111-7. J Immunol. 1996. PMID: 8617931
-
Identification of select lymphocyte homing molecules and vascular addressins in lymphotoxin-alpha deficient mice.Lab Anim. 2000 Jan;34(1):111-4. doi: 10.1258/002367700780578064. Lab Anim. 2000. PMID: 10759375
-
Alpha 4 integrin antagonists.Curr Pharm Des. 2002;8(14):1229-53. doi: 10.2174/1381612023394737. Curr Pharm Des. 2002. PMID: 12052218 Review.
-
Immunity in vaginal candidiasis.Curr Opin Infect Dis. 2005 Apr;18(2):107-11. doi: 10.1097/01.qco.0000160897.74492.a3. Curr Opin Infect Dis. 2005. PMID: 15735412 Review.
Cited by
-
Vaginal and oral epithelial cell anti-Candida activity.Infect Immun. 2002 Dec;70(12):7081-8. doi: 10.1128/IAI.70.12.7081-7088.2002. Infect Immun. 2002. PMID: 12438389 Free PMC article.
-
Role for dendritic cells in immunoregulation during experimental vaginal candidiasis.Infect Immun. 2006 Jun;74(6):3213-21. doi: 10.1128/IAI.01824-05. Infect Immun. 2006. PMID: 16714548 Free PMC article.
-
IL-22 and IDO1 affect immunity and tolerance to murine and human vaginal candidiasis.PLoS Pathog. 2013;9(7):e1003486. doi: 10.1371/journal.ppat.1003486. Epub 2013 Jul 11. PLoS Pathog. 2013. PMID: 23853597 Free PMC article.
-
Animal models of mucosal Candida infection.FEMS Microbiol Lett. 2008 Jun;283(2):129-39. doi: 10.1111/j.1574-6968.2008.01160.x. Epub 2008 Apr 16. FEMS Microbiol Lett. 2008. PMID: 18422625 Free PMC article. Review.
-
Characterization of CD8+ T cells and microenvironment in oral lesions of human immunodeficiency virus-infected persons with oropharyngeal candidiasis.Infect Immun. 2005 Jun;73(6):3659-67. doi: 10.1128/IAI.73.6.3659-3667.2005. Infect Immun. 2005. PMID: 15908395 Free PMC article.
References
-
- Austrup F, Rebstock S, Kilshaw P J, Hamann A. Transforming growth factor-β1-induced expression of the mucosa-related integrin αE on lymphocytes is not associated with mucosa-specific homing. Eur J Immunol. 1995;25:1487–1491. - PubMed
-
- Black C A, Rohan L C, Cost M, Watkins S C, Draviam R, Alber S, Edwards R P. Vaginal mucosa serves as an inductive site for tolerance. J Immunol. 2000;165:5077–5083. - PubMed
-
- Cenci E, Mencacci A, Spaccapelo R, Tonnetti L, Mosci P, Enssle K H, Puccetti P, Romani L, Bistoni F. T helper cell type 1 (Th1)- and Th2-like responses are present in mice with gastric candidiasis but protective immunity is associated with Th1 development. J Infect Dis. 1995;171:1279–1288. - PubMed
-
- Fidel P L, Jr, Ginsburg K A, Cutright J L, Wolf N A, Leaman D, Dunlap K, Sobel J D. Vaginal-associated immunity in women with recurrent vulvovaginal candidiasis: evidence for vaginal Th1-type responses following intravaginal challenge with Candida antigen. J Infect Dis. 1997;176:728–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

