Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae
- PMID: 11447191
- PMCID: PMC98605
- DOI: 10.1128/IAI.69.8.5098-5106.2001
Contribution of Mn-cofactored superoxide dismutase (SodA) to the virulence of Streptococcus agalactiae
Abstract
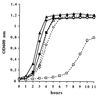
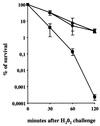
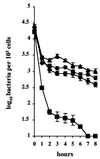
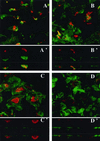
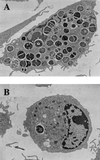
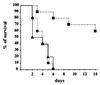
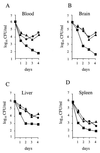
Superoxide dismutases convert superoxide anions to molecular oxygen and hydrogen peroxide, which, in turn, is metabolized by catalases and/or peroxidases. These enzymes constitute one of the major defense mechanisms of cells against oxidative stress and hence play a role in the pathogenesis of certain bacteria. We previously demonstrated that group B streptococci (GBS) possess a single Mn-cofactored superoxide dismutase (SodA). To analyze the role of this enzyme in the pathogenicity of GBS, we constructed a sodA-disrupted mutant of Streptococcus agalactiae NEM316 by allelic exchange. This mutant was subsequently cis complemented by integration into the chromosome of pAT113/Sp harboring the wild-type sodA gene. The SOD specific activity detected by gel analysis in cell extracts confirmed that active SODs were present in the parental and complemented strains but absent in the sodA mutant. The growth rates of these strains in standing cultures were comparable, but the sodA mutant was extremely susceptible to the oxidative stress generated by addition of paraquat or hydrogen peroxide to the culture medium and exhibited a higher mutation frequency in the presence of rifampin. In mouse bone marrow-derived macrophages, the sodA mutant showed an increased susceptibility to bacterial killing by macrophages. In a mouse infection model, after intravenous injection the survival of the sodA mutant in the blood and the brain was markedly reduced in comparison to that of the parental and complemented strains whereas only minor effects on survival in the liver and the spleen were observed. These results suggest that SodA plays a role in GBS pathogenesis.
Figures

References
-
- Baker C J, Edwards M S. Group B streptococcal infections. In: Remington J S, Klein J O, editors. Infectious diseases of the fetus and newborn infant. Philadelphia, Pa: The W. B. Saunders Co; 1990. pp. 743–811.
-
- Bannister J V, Bannister W H, Rotilio G. Aspects of the structure, function and applications of superoxide dismutase. Crit Rev Biochem. 1987;22:111–180. - PubMed
-
- Beauchamp C, Fridovitch I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

