A role for CD36 in the regulation of dendritic cell function
- PMID: 11447263
- PMCID: PMC37507
- DOI: 10.1073/pnas.151028698
A role for CD36 in the regulation of dendritic cell function
Abstract
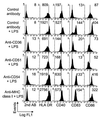
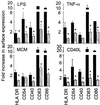
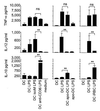
Dendritic cells (DC) are crucial for the induction of immune responses and thus an inviting target for modulation by pathogens. We have previously shown that Plasmodium falciparum-infected erythrocytes inhibit the maturation of DCs. Intact P. falciparum-infected erythrocytes can bind directly to CD36 and indirectly to CD51. It is striking that these receptors, at least in part, also mediate the phagocytosis of apoptotic cells. Here we show that antibodies against CD36 or CD51, as well as exposure to early apoptotic cells, profoundly modulate DC maturation and function in response to inflammatory signals. Although modulated DCs still secrete tumor necrosis factor-alpha, they fail to activate T cells and now secrete IL-10. We therefore propose that intact P. falciparum-infected erythrocytes and apoptotic cells engage similar pathways regulating DC function. These findings may have important consequences for the treatment of malaria and may suggest strategies for modulating pathological immune responses in autoimmune diseases.
Figures

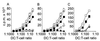

Similar articles
-
CD36 contributes to malaria parasite-induced pro-inflammatory cytokine production and NK and T cell activation by dendritic cells.PLoS One. 2013 Oct 28;8(10):e77604. doi: 10.1371/journal.pone.0077604. eCollection 2013. PLoS One. 2013. PMID: 24204889 Free PMC article.
-
Inhibition of dendritic cell maturation by malaria is dose dependent and does not require Plasmodium falciparum erythrocyte membrane protein 1.Infect Immun. 2007 Jul;75(7):3621-32. doi: 10.1128/IAI.00095-07. Epub 2007 Apr 30. Infect Immun. 2007. PMID: 17470539 Free PMC article.
-
Atypical activation of dendritic cells by Plasmodium falciparum.Proc Natl Acad Sci U S A. 2017 Dec 5;114(49):E10568-E10577. doi: 10.1073/pnas.1708383114. Epub 2017 Nov 21. Proc Natl Acad Sci U S A. 2017. PMID: 29162686 Free PMC article.
-
Macrophage-derived dendritic cells have strong Th1-polarizing potential mediated by beta-chemokines rather than IL-12.J Immunol. 2000 Oct 15;165(8):4388-96. doi: 10.4049/jimmunol.165.8.4388. J Immunol. 2000. PMID: 11035076
-
Dendritic cells in innate immune responses against HIV.Curr Mol Med. 2002 Dec;2(8):739-56. doi: 10.2174/1566524023361907. Curr Mol Med. 2002. PMID: 12462394 Review.
Cited by
-
Apoptosis and dysfunction of blood dendritic cells in patients with falciparum and vivax malaria.J Exp Med. 2013 Jul 29;210(8):1635-46. doi: 10.1084/jem.20121972. Epub 2013 Jul 8. J Exp Med. 2013. PMID: 23835848 Free PMC article.
-
Effect of acute heat stress on adrenocorticotropic hormone, cortisol, interleukin-2, interleukin-12 and apoptosis gene expression in rats.Biomed Rep. 2015 May;3(3):425-429. doi: 10.3892/br.2015.445. Epub 2015 Mar 20. Biomed Rep. 2015. PMID: 26137249 Free PMC article.
-
Keratin mediates the recognition of apoptotic and necrotic cells through dendritic cell receptor DEC205/CD205.Proc Natl Acad Sci U S A. 2016 Nov 22;113(47):13438-13443. doi: 10.1073/pnas.1609331113. Epub 2016 Nov 7. Proc Natl Acad Sci U S A. 2016. PMID: 27821726 Free PMC article.
-
Malaria blood stage suppression of liver stage immunity by dendritic cells.J Exp Med. 2003 Jan 20;197(2):143-51. doi: 10.1084/jem.20021072. J Exp Med. 2003. PMID: 12538654 Free PMC article.
-
CD36 contributes to malaria parasite-induced pro-inflammatory cytokine production and NK and T cell activation by dendritic cells.PLoS One. 2013 Oct 28;8(10):e77604. doi: 10.1371/journal.pone.0077604. eCollection 2013. PLoS One. 2013. PMID: 24204889 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

