Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice
- PMID: 11447267
- PMCID: PMC37512
- DOI: 10.1073/pnas.151179498
Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice
Abstract
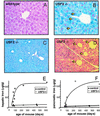
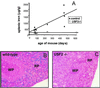
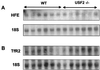
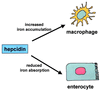
We previously reported the disruption of the murine gene encoding the transcription factor USF2 and its consequences on glucose-dependent gene regulation in the liver. We report here a peculiar phenotype of Usf2(-/-) mice that progressively develop multivisceral iron overload; plasma iron overcomes transferrin binding capacity, and nontransferrin-bound iron accumulates in various tissues including pancreas and heart. In contrast, the splenic iron content is strikingly lower in knockout animals than in controls. To identify genes that may account for the abnormalities of iron homeostasis in Usf2(-/-) mice, we used suppressive subtractive hybridization between livers from Usf2(-/-) and wild-type mice. We isolated a cDNA encoding a peptide, hepcidin (also referred to as LEAP-1, for liver-expressed antimicrobial peptide), that was very recently purified from human blood ultrafiltrate and from urine as a disulfide-bonded peptide exhibiting antimicrobial activity. Accumulation of iron in the liver has been recently reported to up-regulate hepcidin expression, whereas our data clearly show that a complete defect in hepcidin expression is responsible for progressive tissue iron overload. The striking similarity of the alterations in iron metabolism between HFE knockout mice, a murine model of hereditary hemochromatosis, and the Usf2(-/-) hepcidin-deficient mice suggests that hepcidin may function in the same regulatory pathway as HFE. We propose that hepcidin acts as a signaling molecule that is required in conjunction with HFE to regulate both intestinal iron absorption and iron storage in macrophages.
Figures


Comment in
-
Hepcidin: a putative iron-regulatory hormone relevant to hereditary hemochromatosis and the anemia of chronic disease.Proc Natl Acad Sci U S A. 2001 Jul 17;98(15):8160-2. doi: 10.1073/pnas.161296298. Proc Natl Acad Sci U S A. 2001. PMID: 11459944 Free PMC article. Review. No abstract available.
References
-
- Andrews N C. Nat Rev Genet. 2000;1:208–217. - PubMed
-
- Fleming M D, Trenor C C, Su M A, Foernzler D, Beier D R, Dietrich W F, Andrews N C. Nat Genet. 1997;16:383–386. - PubMed
-
- Gunshin H, Mackenzie B, Berger U V, Gunshin Y, Romero M F, Boron W F, Nussberger S, Gollan J L, Hediger M A. Nature (London) 1997;388:482–488. - PubMed
-
- Donovan A, Brownlie A, Zhou Y, Shepard J, Pratt S J, Moynihan J, Paw B H, Drejer A, Barut B, Zapata A, et al. Nature (London) 2000;403:776–781. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

