Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways
- PMID: 11447289
- PMCID: PMC37478
- DOI: 10.1073/pnas.161259898
Critical role for the docking-protein FRS2 alpha in FGF receptor-mediated signal transduction pathways
Abstract
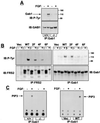
The docking protein FRS2 alpha has been implicated as a mediator of signaling via fibroblast growth factor receptors (FGFRs). We have demonstrated that targeted disruption of FRS2 alpha gene causes severe impairment in mouse development resulting in embryonal lethality at E7.0--E7.5. Experiments with FRS2 alpha-deficient fibroblasts demonstrate that FRS2 alpha plays a critical role in FGF-induced mitogen-activated protein (MAP) kinase stimulation, phosphatidylinositol-3 (PI-3) kinase activation, chemotactic response, and cell proliferation. Following FGF stimulation, tyrosine phosphorylated FRS2 alpha functions as a site for coordinated assembly of a multiprotein complex that includes Gab1 and the effector proteins that are recruited by this docking protein. Furthermore, we demonstrate that different tyrosine phosphorylation sites on FRS2 alpha are responsible for mediating different FGF-induced biological responses. These experiments establish the central role of FRS2 alpha in signaling via FGFRs and demonstrate that FRS2 alpha mediates multiple FGFR-dependent signaling pathways critical for embryonic development.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

