The generality of DNA-templated synthesis as a basis for evolving non-natural small molecules
- PMID: 11448217
- PMCID: PMC2820563
- DOI: 10.1021/ja015873n
The generality of DNA-templated synthesis as a basis for evolving non-natural small molecules
Figures




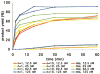
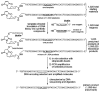

References
-
- Xu Y, Karalkar NB, Kool ET. Nature Biotechnol. 2001;19:148–152. - PubMed
- Luo P, Leitzel JC, Zhan ZYJ, Lynn DG. J Am Chem Soc. 1998;120:3019–3031.
- Herrlein MK, Nelson JS, Letsinger RL. J Am Chem Soc. 1995;117:10151–10152.
- Bruick RK, Dawson PE, Kent SBH, Usman N, Joyce GF. Chem Biol. 1996;3:49–56. - PubMed
- Gat Y, Lynn DG. Biopolymers. 1998;48:19–28. - PubMed
- Orgel LE. Acc Chem Res. 1995;28:109–118. - PubMed
- Luther A, Brandsch R, von Kiedrowski G. Nature. 1998;396:245–8. - PubMed
-
- Meinkoth J, Wahl GM. Anal Biochem. 1984;138:267–284. - PubMed
-
- Illuminati G, Mandolini L. Acc Chem Res. 1981;14:95–102.
- Woodward RB, Logusch E, Nambiar KP, Sakan K, Ward DE, Auyeung BW, Balaram P, Browne LJ, Card PJ, Chen CH, Chenevert RB, Fliri A, Frobel K, Gais HJ, Garratt DG, Hayakawa K, Heggie W, Hesson DP, Hoppe D, Hoppe I, Hyatt JA, Ikeda D, Jacobi PA, Kim KS, Kobuke Y, Kojima K, Krowicki K, Lee VJ, Leutert T, Malchenko S, Martens J, Matthews RS, Ong BS, Press JB, Babu TVR, Rousseau G, Sauter HM, Suzuki M, Tatsuta K, Tolbert LM, Truesdale EA, Uchida I, Ueda Y, Uyehara T, Vasella AT, Vladuchick WC, Wade PA, Williams RM, Wong HNC. J Am Chem Soc. 1981;103:3210–3213.
-
- Li CJ, Chan TH. Organic Reactions in Aqueous Media. Wiley and Sons; New York: 1997.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
