Human Vam6p promotes lysosome clustering and fusion in vivo
- PMID: 11448994
- PMCID: PMC2196876
- DOI: 10.1083/jcb.200102142
Human Vam6p promotes lysosome clustering and fusion in vivo
Abstract
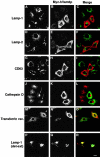
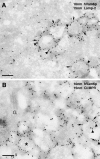
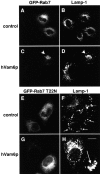
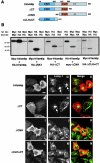
Regulated fusion of mammalian lysosomes is critical to their ability to acquire both internalized and biosynthetic materials. Here, we report the identification of a novel human protein, hVam6p, that promotes lysosome clustering and fusion in vivo. Although hVam6p exhibits homology to the Saccharomyces cerevisiae vacuolar protein sorting gene product Vam6p/Vps39p, the presence of a citron homology (CNH) domain at the NH(2) terminus is unique to the human protein. Overexpression of hVam6p results in massive clustering and fusion of lysosomes and late endosomes into large (2-3 microm) juxtanuclear structures. This effect is reminiscent of that caused by expression of a constitutively activated Rab7. However, hVam6p exerts its effect even in the presence of a dominant-negative Rab7, suggesting that it functions either downstream of, or in parallel to, Rab7. Data from gradient fractionation, two-hybrid, and coimmunoprecipitation analyses suggest that hVam6p is a homooligomer, and that its self-assembly is mediated by a clathrin heavy chain repeat domain in the middle of the protein. Both the CNH and clathrin heavy chain repeat domains are required for induction of lysosome clustering and fusion. This study implicates hVam6p as a mammalian tethering/docking factor characterized with intrinsic ability to promote lysosome fusion in vivo.
Figures






References
-
- Aguilar, R.C., H. Ohno, K.W. Roche, and J.S. Bonifacino. 1997. Functional mapping of the clathrin-associated adaptor medium chains μ1 and μ2. J. Biol. Chem. 272:27160–27166. - PubMed
-
- Bakker, A.C., P. Webster, W.A. Jacob, and N.W. Andrews. 1997. Homotypic fusion between aggregated lysosomes triggered by elevated [Ca2+]i in fibroblasts. J. Cell Sci. 110:2227–2238. - PubMed
-
- Caplan, S., E.C. Dell'Angelica, W.A. Gahl, and J.S. Bonifacino. 2000. Trafficking of major histocompatibility complex class II molecules in human B-lymphoblasts deficient in the AP-3 adaptor complex. Immunol. Lett. 72:113–117. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

