Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly
- PMID: 11448995
- PMCID: PMC2196875
- DOI: 10.1083/jcb.200102110
Obscurin, a giant sarcomeric Rho guanine nucleotide exchange factor protein involved in sarcomere assembly
Abstract
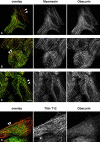
Vertebrate-striated muscle is assumed to owe its remarkable order to the molecular ruler functions of the giant modular signaling proteins, titin and nebulin. It was believed that these two proteins represented unique results of protein evolution in vertebrate muscle. In this paper we report the identification of a third giant protein from vertebrate muscle, obscurin, encoded on chromosome 1q42. Obscurin is approximately 800 kD and is expressed specifically in skeletal and cardiac muscle. The complete cDNA sequence of obscurin reveals a modular architecture, consisting of >67 intracellular immunoglobulin (Ig)- or fibronectin-3-like domains with multiple splice variants. A large region of obscurin shows a modular architecture of tandem Ig domains reminiscent of the elastic region of titin. The COOH-terminal region of obscurin interacts via two specific Ig-like domains with the NH(2)-terminal Z-disk region of titin. Both proteins coassemble during myofibrillogenesis. During the progression of myofibrillogenesis, all obscurin epitopes become detectable at the M band. The presence of a calmodulin-binding IQ motif, and a Rho guanine nucleotide exchange factor domain in the COOH-terminal region suggest that obscurin is involved in Ca(2+)/calmodulin, as well as G protein-coupled signal transduction in the sarcomere.
Figures




Comment in
-
Fishing out proteins that bind to titin.J Cell Biol. 2001 Jul 9;154(1):21-4. doi: 10.1083/jcb.200106072. J Cell Biol. 2001. PMID: 11448986 Free PMC article. Review.
Similar articles
-
The complete gene sequence of titin, expression of an unusual approximately 700-kDa titin isoform, and its interaction with obscurin identify a novel Z-line to I-band linking system.Circ Res. 2001 Nov 23;89(11):1065-72. doi: 10.1161/hh2301.100981. Circ Res. 2001. PMID: 11717165
-
The rho-guanine nucleotide exchange factor domain of obscurin regulates assembly of titin at the Z-disk through interactions with Ran binding protein 9.Mol Biol Cell. 2008 Sep;19(9):3782-92. doi: 10.1091/mbc.e08-03-0237. Epub 2008 Jun 25. Mol Biol Cell. 2008. PMID: 18579686 Free PMC article.
-
Identification, tissue expression and chromosomal localization of human Obscurin-MLCK, a member of the titin and Dbl families of myosin light chain kinases.Gene. 2002 Jan 9;282(1-2):237-46. doi: 10.1016/s0378-1119(01)00795-8. Gene. 2002. PMID: 11814696
-
Obscurin: a multitasking muscle giant.J Muscle Res Cell Motil. 2005;26(6-8):419-26. doi: 10.1007/s10974-005-9024-7. J Muscle Res Cell Motil. 2005. PMID: 16625317 Review.
-
Muscle giants: molecular scaffolds in sarcomerogenesis.Physiol Rev. 2009 Oct;89(4):1217-67. doi: 10.1152/physrev.00017.2009. Physiol Rev. 2009. PMID: 19789381 Free PMC article. Review.
Cited by
-
The sarcomeric Z-disc: a nodal point in signalling and disease.J Mol Med (Berl). 2006 Jun;84(6):446-68. doi: 10.1007/s00109-005-0033-1. Epub 2006 Jan 17. J Mol Med (Berl). 2006. PMID: 16416311 Review.
-
Sel1 repeat protein LpnE is a Legionella pneumophila virulence determinant that influences vacuolar trafficking.Infect Immun. 2007 Dec;75(12):5575-85. doi: 10.1128/IAI.00443-07. Epub 2007 Sep 24. Infect Immun. 2007. PMID: 17893138 Free PMC article.
-
Titin/connectin-related proteins in C. elegans: a review and new findings.J Muscle Res Cell Motil. 2005;26(6-8):435-47. doi: 10.1007/s10974-005-9027-4. J Muscle Res Cell Motil. 2005. PMID: 16453163 Review. No abstract available.
-
Functional properties of the titin/connectin-associated proteins, the muscle-specific RING finger proteins (MURFs), in striated muscle.J Muscle Res Cell Motil. 2005;26(6-8):389-400. doi: 10.1007/s10974-005-9021-x. J Muscle Res Cell Motil. 2005. PMID: 16477476
-
The role of the M-band myomesin proteins in muscle integrity and cardiac disease.J Biomed Sci. 2022 Mar 7;29(1):18. doi: 10.1186/s12929-022-00801-6. J Biomed Sci. 2022. PMID: 35255917 Free PMC article. Review.
References
-
- Aghazadeh, B., W.E. Lowry, X.-Y. Huang, and M.K. Rosen. 2000. Structural basis for relief of autoinhibition of the Dbl homology domain of proto-oncogene Vav by tyrosine phosphorylation. Cell. 102:625–633. - PubMed
-
- Andersson, S., D.N. Davis, H. Dahlbäck, H. Jörnvall, and D.W. Russell. 1989. Cloning, structure, and expression of the mitochondrial cytochrome P-450 sterol 26-hydroxylase, a bile acid biosynthetic enzyme. J. Biol. Chem. 264:8222–8229. - PubMed
-
- Ausubel, F.M., R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl. 1995. Short protocols in molecular biology. Wiley and Sons, Inc., New York.
-
- Benian, G.M., J.E. Kiff, N. Neckelmann, D.G. Moerman, and R.H. Waterston. 1989. Sequence of an unusually large protein implicated in regulation of myosin activity in C. elegans. Nature. 342:45–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

