One plant actin isovariant, ACT7, is induced by auxin and required for normal callus formation
- PMID: 11449050
- PMCID: PMC139544
- DOI: 10.1105/tpc.010026
One plant actin isovariant, ACT7, is induced by auxin and required for normal callus formation
Abstract
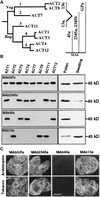
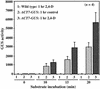
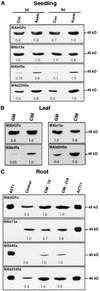
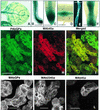
During plant growth and development, the phytohormone auxin induces a wide array of changes that include cell division, cell expansion, cell differentiation, and organ initiation. It has been suggested that the actin cytoskeleton plays an active role in the elaboration of these responses by directing specific changes in cell morphology and cytoarchitecture. Here we demonstrate that the promoter and the protein product of one of the Arabidopsis vegetative actin genes, ACT7, are rapidly and strongly induced in response to exogenous auxin in the cultured tissues of Arabidopsis. Homozygous act7-1 mutant plants were slow to produce callus tissue in response to hormones, and the mutant callus contained at least two to three times lower levels of ACT7 protein than did the wild-type callus. On the other hand, a null mutation in ACT2, another vegetative actin gene, did not significantly affect callus formation from leaf or root tissue. Complementation of the act7-1 mutants with the ACT7 genomic sequence restored their ability to produce callus at rates similar to those of wild-type plants, confirming that the ACT7 gene is required for callus formation. Immunolabeling of callus tissue with actin subclass-specific antibodies revealed that the predominant ACT7 is coexpressed with the other actin proteins. We suggest that the coexpression, and probably the copolymerization, of the abundant ACT7 with the other actin isovariants in cultured cells may facilitate isovariant dynamics well suited for cellular responses to external stimuli such as hormones.
Figures





Similar articles
-
A single vegetative actin isovariant overexpressed under the control of multiple regulatory sequences is sufficient for normal Arabidopsis development.Plant Cell. 2009 Mar;21(3):701-18. doi: 10.1105/tpc.108.061960. Epub 2009 Mar 20. Plant Cell. 2009. PMID: 19304937 Free PMC article.
-
Actin Isovariant ACT7 Modulates Root Thermomorphogenesis by Altering Intracellular Auxin Homeostasis.Int J Mol Sci. 2021 Jul 20;22(14):7749. doi: 10.3390/ijms22147749. Int J Mol Sci. 2021. PMID: 34299366 Free PMC article.
-
Actin isovariant ACT7 controls root meristem development in Arabidopsis through modulating auxin and ethylene responses.J Exp Bot. 2022 Oct 18;73(18):6255-6271. doi: 10.1093/jxb/erac280. J Exp Bot. 2022. PMID: 35749807
-
A Common Molecular Signature Indicates the Pre-Meristematic State of Plant Calli.Int J Mol Sci. 2023 Aug 23;24(17):13122. doi: 10.3390/ijms241713122. Int J Mol Sci. 2023. PMID: 37685925 Free PMC article. Review.
-
Critical role of actin bundling in plant cell morphogenesis.Plant Signal Behav. 2010 May;5(5):484-8. doi: 10.4161/psb.10947. Epub 2010 Feb 1. Plant Signal Behav. 2010. PMID: 20118661 Free PMC article. Review.
Cited by
-
Plant pathogenic bacteria target the actin microfilament network involved in the trafficking of disease defense components.Bioarchitecture. 2014;4(4-5):149-53. doi: 10.4161/19490992.2014.980662. Bioarchitecture. 2014. PMID: 25551177 Free PMC article.
-
CINCINNATA-Like TCP Transcription Factors in Cell Growth - An Expanding Portfolio.Front Plant Sci. 2022 Feb 22;13:825341. doi: 10.3389/fpls.2022.825341. eCollection 2022. Front Plant Sci. 2022. PMID: 35273626 Free PMC article. Review.
-
Plant Rac-like GTPases are activated by auxin and mediate auxin-responsive gene expression.Plant Cell. 2002 Nov;14(11):2745-60. doi: 10.1105/tpc.006320. Plant Cell. 2002. PMID: 12417698 Free PMC article.
-
Bundling up the Role of the Actin Cytoskeleton in Primary Root Growth.Front Plant Sci. 2021 Dec 16;12:777119. doi: 10.3389/fpls.2021.777119. eCollection 2021. Front Plant Sci. 2021. PMID: 34975959 Free PMC article. Review.
-
NaCl-responsive ROS scavenging and energy supply in alkaligrass callus revealed from proteomic analysis.BMC Genomics. 2019 Dec 17;20(1):990. doi: 10.1186/s12864-019-6325-6. BMC Genomics. 2019. PMID: 31847807 Free PMC article.
References
-
- Abel, S., Ballas, N., Wong, L.M., and Theologis, A. (1996). DNA elements responsive to auxin. Bioessays 18, 647–654. - PubMed
-
- An, Y.-Q., McDowell, J.M., Huang, S., McKinney, E.C., Chambliss, S., and Meagher, R.B. (1996. b). Strong, constitutive expression of the Arabidopsis ACT2/ACT8 actin subclass in vegetative tissues. Plant J. 10, 107–121. - PubMed
-
- Brummell, D.A., and Hall, J.L. (1987). Rapid cellular responses to auxin and the regulation of growth. Plant Cell Environ. 10, 523–543.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

