Overexpression of the heterotrimeric G-protein alpha-subunit enhances phytochrome-mediated inhibition of hypocotyl elongation in Arabidopsis
- PMID: 11449056
- PMCID: PMC139542
- DOI: 10.1105/tpc.010008
Overexpression of the heterotrimeric G-protein alpha-subunit enhances phytochrome-mediated inhibition of hypocotyl elongation in Arabidopsis
Abstract
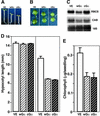
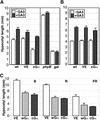
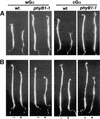
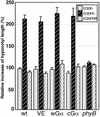
Plant heterotrimeric G-proteins have been implicated in a number of signaling processes. However, most of these studies are based on biochemical or pharmacological approaches. To examine the role of heterotrimeric G-proteins in plant development, we generated transgenic Arabidopsis expressing the Galpha subunit of the heterotrimeric G-protein under the control of a glucocorticoid-inducible promoter. With the conditional overexpression of either the wild type or a constitutively active version of Arabidopsis Galpha, transgenic seedlings exhibited a hypersensitive response to light. This enhanced light sensitivity was more exaggerated in a relatively lower intensity of light and was observed in white light as well as far-red, red, and blue light conditions. The enhanced responses in far-red and red light required functional phytochrome A and phytochrome B, respectively. Furthermore, the response to far-red light depended on functional FHY1 but not on FIN219 and FHY3. This dependence on FHY1 indicates that the Arabidopsis Galpha protein may act only on a discrete branch of the phytochrome A signaling pathway. Thus, our results support the involvement of a heterotrimeric G-protein in the light regulation of Arabidopsis seedling development.
Figures






Similar articles
-
A reevaluation of the role of the heterotrimeric G protein in coupling light responses in Arabidopsis.Plant Physiol. 2003 Apr;131(4):1623-7. doi: 10.1104/pp.102.017624. Plant Physiol. 2003. PMID: 12692321 Free PMC article.
-
Discrete and essential roles of the multiple domains of Arabidopsis FHY3 in mediating phytochrome A signal transduction.Plant Physiol. 2008 Oct;148(2):981-92. doi: 10.1104/pp.108.120436. Epub 2008 Aug 20. Plant Physiol. 2008. PMID: 18715961 Free PMC article.
-
FHL is required for full phytochrome A signaling and shares overlapping functions with FHY1.Plant J. 2005 Aug;43(3):356-70. doi: 10.1111/j.1365-313X.2005.02453.x. Plant J. 2005. PMID: 16045472 Free PMC article.
-
Heterotrimeric G-proteins in plant development.Front Biosci. 2008 May 1;13:3321-33. doi: 10.2741/2928. Front Biosci. 2008. PMID: 18508435 Review.
-
Plant G proteins: the different faces of GPA1.Curr Biol. 2001 Oct 30;11(21):R869-71. doi: 10.1016/s0960-9822(01)00519-x. Curr Biol. 2001. PMID: 11696344 Review.
Cited by
-
Chemically regulated expression systems and their applications in transgenic plants.Transgenic Res. 2003 Oct;12(5):529-40. doi: 10.1023/a:1025852307127. Transgenic Res. 2003. PMID: 14601652 Review.
-
Phytochrome signaling mechanisms.Arabidopsis Book. 2011;9:e0148. doi: 10.1199/tab.0148. Epub 2011 Aug 29. Arabidopsis Book. 2011. PMID: 22303272 Free PMC article.
-
HYPERSENSITIVE TO RED AND BLUE 1, a ZZ-type zinc finger protein, regulates phytochrome B-mediated red and cryptochrome-mediated blue light responses.Plant Cell. 2005 Mar;17(3):822-35. doi: 10.1105/tpc.104.029165. Epub 2005 Feb 10. Plant Cell. 2005. PMID: 15705950 Free PMC article.
-
Characterization of a strong dominant phytochrome A mutation unique to phytochrome A signal propagation.Plant Physiol. 2002 Sep;130(1):457-65. doi: 10.1104/pp.005264. Plant Physiol. 2002. PMID: 12226524 Free PMC article.
-
GCR1, the putative Arabidopsis G protein-coupled receptor gene is cell cycle-regulated, and its overexpression abolishes seed dormancy and shortens time to flowering.Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4736-41. doi: 10.1073/pnas.072087699. Proc Natl Acad Sci U S A. 2002. PMID: 11930019 Free PMC article.
References
-
- Aharon, G., Gelli, A., Snedden, W.A., and Blumwald, E. (1998). Activation of a plant plasma membrane Ca2+ channel by TGα1, a heterotrimeric G protein α-subunit homologue. FEBS Lett. 424, 17–21. - PubMed
-
- Aoyama, T., and Chua, N.-H. (1997). A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11, 605–612. - PubMed
-
- Arabidopsis Genome Initiative. (2000). Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408, 796–815. - PubMed
-
- Assmann, S.M. (1996). Guard cell G proteins. Trends Plant Sci. 1, 73–74.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

